The techniques used to separate the substances that make up the homogeneous and heterogeneous mixtures take into account the number of phases, the nature of the components and their properties.
Use the following questions to test your knowledge and complement your studies with the commented resolution.
question 1
Relate the method of separation of mixtures to the property used in the separation process.
I. Decantation
II. Distillation
III. Solvent Extraction
THE. Solubility
B. Density
Ç. boiling temperature
Correct answer: I.B; II.C. and III.A.
I.B: A decant is a process of separation of heterogeneous mixtures, where natural separation occurs through the density of the immiscible components of a solid-liquid or liquid-liquid mixture.
For example, in a water treatment tank, solid materials settle and settle to the bottom because they are denser.
II.C: A distillation is a process of separation of mixtures, whose components have different boiling temperatures.
For example, a component of a mixture that has a low boiling point tends to go into a gaseous state first and thus is separated from the others. The separated component can then be condensed (returned to a liquid) and collected.
III.A: A solvent extraction is performed in accordance with the solubility of the compounds in a mixture.
For example, when we prepare coffee, we use hot water in the powder, which drags the soluble compounds with it. This is because coffee has substances that are soluble in hot water.
question 2
Review the following statements and judge as true (T) or false (F).
( ) In filtration, solid and liquid are separated by the difference in particle size.
( ) Centrifugation is a decantation process accelerated by the force of gravity.
( ) The separation in chromatography is carried out by the interaction of the mixture components with the mobile phase and the stationary phase.
Correct answer: V; F; V.
(V) In filtration, solid and liquid are separated by the difference in particle size.
Solid particles are separated into a solid-liquid mixture as they are retained in the filter medium, as they are larger in size and cannot exceed the filter pores.
(F) Centrifugation is a decantation process accelerated by the force of gravity.
Centrifugation is carried out by the action of centrifugal force, which accelerates the decantation process by circular movement, causing the denser material to settle on the bottom of the container.
(V) The separation in chromatography is carried out by the interaction of the mixture components with the mobile phase and the stationary phase.
In the chromatography method, the mixture of components is inserted into the stationary phase, a material capable of retaining the substances to be separated. When the mobile phase passes through the compounds, it can drag them through the stationary phase by interacting with them. The greater the interaction of the mobile phase with a component of the mixture, the greater its migration through the stationary phase.
question 3
(Vunesp) When preparing coffee, hot water comes into contact with the powder and is separated in the strainer. The operations involved in this separation are, respectively:
a) distillation and decantation.
b) filtration and distillation.
c) distillation and coercion.
d) extraction and filtration.
e) extraction and decantation.
Correct alternative: d) extraction and filtration.
a) WRONG. Distillation separates two mixed liquids according to their boiling point. Decantation is the separation of a mixture of solid and liquid by gravity.
b) WRONG. Filtration uses a filter medium to retain solids and distillation separates liquids according to boiling point.
c) WRONG. Distillation is used to separate a homogeneous mixture of liquids. Straining is the popular way of calling coffee filtration, which uses a strainer.
d) CORRECT. Solvent extraction is carried out with water that, when in contact with the coffee, solubilizes the components that were in the solid and drags them away. The powder is retained in the strainer, which represents the filter medium for filtration.
e) WRONG. Although extraction is represented by the use of hot water as a solvent, decantation is not part of this process.
know more about Separation of mixtures.
question 4
(Unirio) A mixture formed by gasoline, water, sawdust and table salt can be separated into its various components by following the steps below:
a) filtration, decantation and distillation.
b) collection and decantation.
c) sublimation and distillation.
d) pressing and decanting.
e) distillation and decantation.
Correct alternative: a) filtration, decantation and distillation.
The mixture composed of gasoline, water, sawdust and table salt components has 3 phases:
| Phase 1 | Gasoline |
| Level 2 | sawdust |
| Stage 3 | water and salt |
a) CORRECT. The filtration separates phase 2 from the other phases, as the sawdust, solid particles, are retained in the filter medium.
Decantation separates phase 1 from phase 3, as liquids have different densities and due to their chemical characteristics do not mix, as water is polar and gasoline is non-polar.
The distillation promotes the evaporation of the solvent, the water, and the solid that was dissolved, the table salt, crystallizes out again.
b) WRONG. Scavenging corresponds to the separation of solids with different particle sizes, which is not the case presented in the question. Decantation is only capable of separating the water and gasoline components.
c) WRONG. Sublimation is not a process of separation of mixtures, but a change in physical state, which corresponds to the passage from a solid to a gaseous state, without going through the liquid state. Distillation is only capable of separating the water and table salt components.
d) WRONG. Pressing corresponds to a physical operation of compressing an object, which does not apply to the case presented in the question. Decantation is only capable of separating the water and gasoline components.
e) WRONG. Distillation separates table salt from water, which is evaporated in the process. Decantation removes gasoline due to the difference in density with water. However, the two operations alone are not enough to separate all the components, as the sawdust would still be missing.
question 5
(Unicid) Number the second column according to the first, then choose the option corresponding to the correct numbering, from top to bottom
| Mixtures | Main methods of separation |
|---|---|
| 1) Oxygen and nitrogen | (///) Distillation |
| 2) Oil and water | (///) Filtration |
| 3) Alcohol and water | (///) Magnetic separation |
| 4) Iron and sulfur | (///) Decanting |
| 5) Air and dust | (///) Liquefaction |
a) 1 - 4 - 5 - 2 - 3
b) 1 - 5 - 4 - 3 - 2
c) 3 - 2 - 4 - 5 -1
d) 3 - 5 - 4 - 2 - 1
e) 5 - 1 - 3 - 4 - 2
Correct alternative: d) 3 - 5 - 4 - 2 - 1.
Distillation separates alcohol and water (3), as the two components have different boiling temperatures.
filtration separates air and dust (5), because due to the size of the particles, the dust is retained in a filter.
magnetic separation separates iron and sulfur (4), as iron has magnetic properties and is attracted by a magnet.
Decantation separates oil and water (2), as the two components of the mixture have different densities and are not miscible.
Liquefaction separates oxygen and nitrogen (1) as the two gases create a homogeneous mixture. In this process, the air is cooled until it becomes liquid. After that, the mixture is heated and the components are separated by boiling point in a distillation column.
question 6
(Cesgranrio) In one of the stages of water treatment that supplies a city, the water is kept for a certain time in tanks so that the suspended solids settle on the bottom. This operation is called:
a) filtration.
b) sedimentation.
c) siphonation.
d) centrifugation.
e) crystallization.
Correct alternative: b) sedimentation.
a) WRONG. This operation separates suspended solids from liquid with a filter paper, which retains the particles while the mixture passes through it.
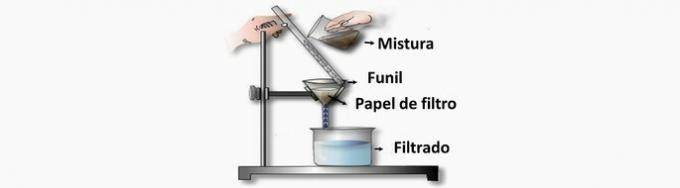
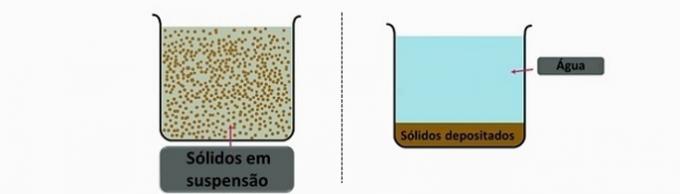
b) CORRECT. This operation, by the action of gravity, makes the denser particles reach the bottom of the tank and settle when the water is at rest.
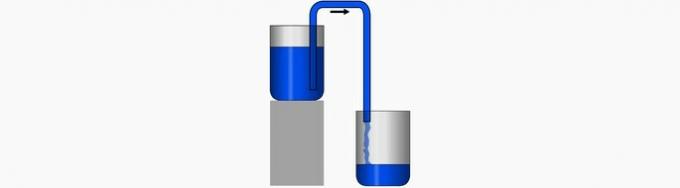
c) WRONG. This operation corresponds to the transport of a liquid from an upper to a lower level, that is, the liquid flows into the lower container.
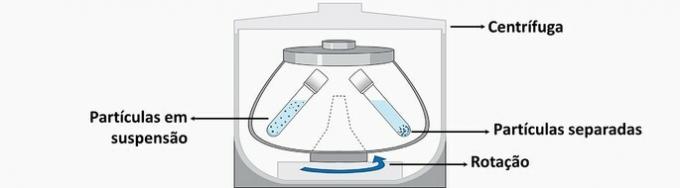
d) WRONG. This operation causes the separation of a mixture to occur by applying the centrifugal force acquired by the rotations of the device.
e) WRONG. This operation performs the separation of solid and liquid by evaporating the solvent. The solid that was dissolved is recrystallized.
To complement the reading, we suggest the textWater treatment.
question 7
(Unifor) A solid A is completely dissolved in a liquid B. It is possible to separate solvent B from the mixture by means of a:
a) centrifugation.
b) siphonation.
c) decanting.
d) filtration.
e) distillation.
Correct alternative: e) distillation.
a) WRONG. Centrifugation separates suspended solids in a liquid.

b) WRONG. Siphoning transports the liquid from a higher point to a lower one.

c) WRONG. Decantation separates components with different densities by the force of gravity.

d) WRONG. Filtration retains solid particles suspended in a liquid using a filter medium.

e) CORRECT. Distillation is able to evaporate solvent B and solid A is crystallized.

question 8
(UnB) Judge the following items, marking C for correct and E for wrong.
1) Evaporation allows the separation of two highly volatile liquids.
2) It is possible to separate a homogeneous liquid-liquid material by fractional distillation.
3) The separation of petroleum components is based on the difference between their respective boiling temperatures.
4) The principle of fractional distillation is based on the difference in solubility of solids in a material.
1) WRONG. Evaporation is a change in physical state, which corresponds to the passage from a liquid to a gaseous state. If the two liquids are highly volatile, then they have very close boiling points, which makes separation difficult.
2) CORRECT. Liquids are separated by different boiling points. The liquid with the lowest boiling point is removed from the system first.
3) CORRECT. Petroleum is a complex mixture of hydrocarbons that have different physical and chemical properties. Distillation towers separate the components of the mixture according to their volatility, as the boiling points vary with the size of the carbon chain.
4) WRONG. It is based on the different boiling points of the mixture components.
question 9
(UFRGS) A biphasic heterogeneous system is formed by three different liquids A, B and C. It is known that:
- A and B are miscible with each other;
- C is immiscible with A and B;
- A is more volatile than B.
Based on this information, the most suitable methods for separating the three liquids are:
a) centrifuging and decanting.
b) decantation and fractional melting.
c) filtration and centrifugation.
d) filtration and fractional distillation.
e) decantation and fractional distillation.
Correct alternative: e) decantation and fractional distillation.
According to the data in the statement, the system presents two phases:
| Phase 1 | Liquids A and B |
| Level 2 | Net C |
a) WRONG. Centrifugation cannot be used as it is suitable for separating particles suspended in a liquid.
b) WRONG. Fractional melting cannot be used as it is suitable for separating solids with different melting points.
c) WRONG. Filtration cannot be used as it is suitable for separating solids into liquids. Likewise, centrifugation is not useful as it is an accelerated solid-liquid separation process.
d) WRONG. Filtration cannot be used as it is useful in separating heterogeneous solid-liquid systems.
e) CORRECT. Decantation promotes the separation of phase 1 with components A and B of phase 2, which corresponds to component C by the difference in density.
Distillation promotes the separation of A and B according to the boiling point of the substances. The mixture is heated and the lower boiling component is first evaporated and collected in another container.
question 10
(Ufba) Based on the diagram below, it is correct to state:

a) Process X is filtration.
b) Solid A is calcium carbonate, CaCO3.
c) Process Y is decanting.
d) System C is a homogeneous material.
e) System D has a substance.
f) Process Z is a simple distillation.
g) Distilled water is a material.
Correct alternatives: a, b, c, f.
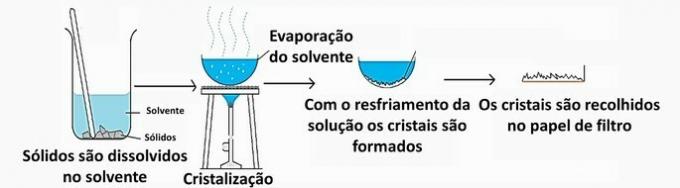
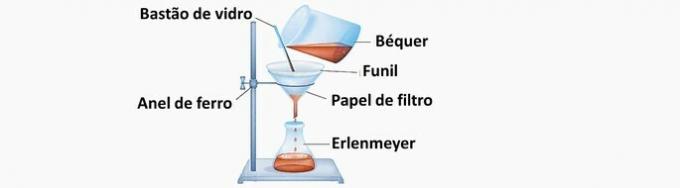
a) CORRECT. Solid A was separated from system B as it was not dissolved therein. The filtration retains the solid particles, which remain on the filter paper, while the liquid that has passed through the funnel is collected in another container.
b) CORRECT. Heating caused a chemical reaction to take place. In it, new substances were formed by the decomposition of calcium carbonate (CaCO3).
CaCO3 CO2(g) + CaO(s)
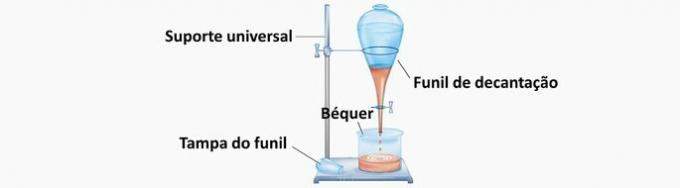
c) CORRECT. As the mixture is composed of immiscible liquids, the separation occurred due to the difference in density. During decantation, using a bromine funnel, the components spontaneously separate.
d) WRONG. If the system is single-phase, it has only one phase and can be a homogeneous mixture or a pure substance.
e) WRONG. System D is a homogeneous mixture of water and calcium carbonate (CaCO) components3).
f) CORRECT. As system D is homogeneous, composed of liquid and solid, through distillation the liquid can be evaporated and subsequently condensed to be collected in another container.

g) WRONG. Distilled water is a substance, as it has fixed and invariable properties throughout its entirety.
question 11
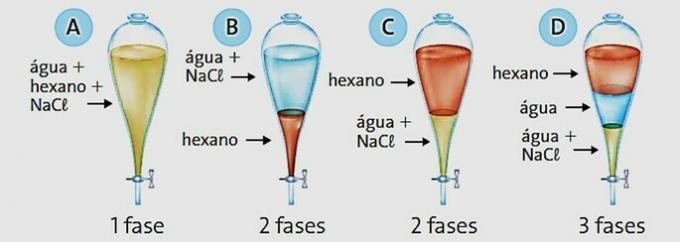
(PUC-MG) When placing hexane (d = 0.66 g/cm3), water (d = 1 g/cm3) and salt (NaCl) in a laboratory glassware known as a separation funnel (picture below), mark the appropriate aspect observed after some time of rest.
a) A
b) B
c) C
d) D
Correct alternative: c) C.
Hexane has a lower density than water, so it is the component at the top of the decanting funnel.
When we put salt in water, the ions dissociate, as shown by the chemical equation:
As it is an ionic and water-soluble compound, sodium chloride undergoes an alteration, breaking down into its ions, thus, water and salt represent a phase in the system.
The result is a product with an intermediate density between that of water and salt, which is 2.16 g/cm³. This is because water is no longer a pure substance and has become a mixture.
Get more knowledge by reading about Physical and Chemical Transformations.
question 12
(Cairu) About materials separation processes, indicate the correct alternative.
a) Straining coffee, a material separation process, is a physical phenomenon.
b) Phase of a system are the components that make up that system.
c) One of the processes often used to separate seawater from salt is filtration.
d) When substances change from solid to liquid, there is evidence that a chemical reaction has occurred.
e) Fractional distillation is a process often used to separate two solids.
Correct alternative: a) Straining coffee, a material separation process, is a physical phenomenon.
a) CORRECT. Straining the coffee is the same as filtering it. So it's a physical phenomenon.
b) WRONG. The phase corresponds to the region of the system that presents the same aspects throughout its extension.
c) WRONG. The process often used is evaporation. Water, which has a lower boiling point than salt, is separated first.
d) WRONG. The change in physical state represents a physical transformation. A chemical reaction takes place when new substances are formed.
e) WRONG. Fractional distillation is often used to separate liquid components from a homogeneous mixture, ie the substances are miscible. Therefore, the distillation separates according to the different boiling points.
See too: Exercises on homogeneous and heterogeneous mixtures.


