Chemical isomerism is a phenomenon observed when two or more organic substances have the same molecular formula, but different molecular structure and properties.
Chemical substances with these characteristics are called isomers.
The term is derived from the Greek words iso = equal and mere = parts, that is, equal parts.
There are different types of isomerism:
- flat isomer: Compounds are identified by flat structural formulas. It is divided into chain isomerism, function isomerism, position isomerism, compensation isomerism and tautomerism isomerism.
- space isomer: The molecular structure of compounds has different spatial structures. It is divided into geometric and optical isomerism.
flat isomer
At flat isomerism or constitutional isomerism, the molecular structure of organic substances is flat.
Compounds that exhibit this characteristic are called flat isomers.
chain isomer
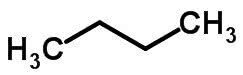
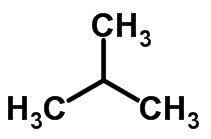
Chain isomerism happens when carbon atoms have different chains and the same chemical function.
Examples:
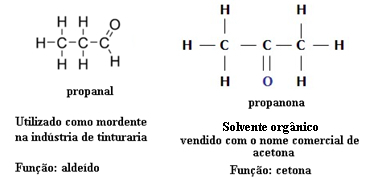
Function Isomerism
Function isomerism occurs when two or more compounds have different chemical functions and the same molecular formula.
Examples: This case is common between aldehydes and ketones.
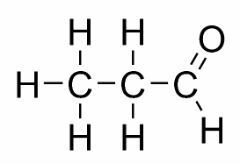
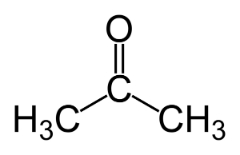
Position isomer
Position isomerism occurs when compounds are differentiated by different unsaturation, branching or functional group positions in the carbon chain. In this case, the isomers have the same chemical function.
Examples:

offsetting isomer
Compensation isomerism or metamerism occurs in compounds with the same chemical function that differ by the position of the heteroatoms.
Examples:

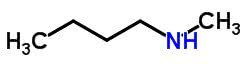
Tautomery
Tautomerism or dynamic isomerism can be considered a specific case of function isomerism. In this case, one isomer can change into another by changing the position of an element in the chain.
Examples:

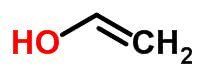
space isomer
THE space isomerism, also called stereoisomerism, happens when two compounds have the same molecular formula and different structural formulas.
In this type of isomerism, atoms are distributed the same way, but occupy different positions in space.
geometric isomer
THE geometric isomerism or cis-trans occurs in unsaturated open chains and also in cyclic compounds. To do this, the carbon ligands must be different.

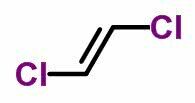
- When the same ligands are on the same side, the isomer nomenclature receives the prefix cis.
- When the same ligands are on opposite sides, the nomenclature receives the prefix trans.
The IUPAC (International Union of Pure and Applied Chemistry) recommends that instead of cis and trans, the letters Z and E be used as a prefix.
This is because Z is the first letter of the German word zusammen, which means "together". And it's the first letter of the German word entegegen, which means "opposite".
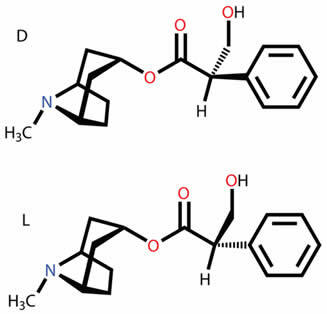
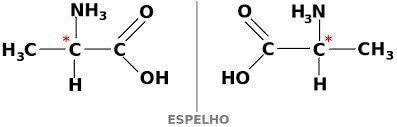
optical isomer
THE optical isomerism is demonstrated by compounds that are optically active. It happens when a substance is caused by the angular deviation in the plane of polarized light.
- When a substance bends optical light to the right it is called right-handed.
- When a substance bends optical light to the left, the substance is called levorotary.
A substance can also exist in two forms that are optically active, dextogyra and levogyra. In this case, it is called enantiomer.
For a carbon compound to be optically active, it must be chiral. This means that their ligands cannot overlap, being asymmetric.
In turn, if a compound has the dextrogyra and levorotary forms in equal parts, they are called racemic mixtures. The optical activity of racemic mixtures is inactive.
Read too:
- Carbon
- Organic chemistry
- Molecular Formula
- structural formula
Exercises
1. (Mackenzie 2012) Number column B, which contains organic compounds, associating them with column A, according to the type of isomerism that each organic molecule presents.
Column A
1. offsetting isomer
2. geometric isomer
3. chain isomer
4. optical isomer
Column B
( ) cyclopropane
( ) ethoxy-ethane
( ) bromo-chloro-fluoro-methane
( ) 1,2-dichloro-ethene
The correct sequence of numbers in column B, from top to bottom, is
a) 2 - 1 - 4 - 3.
b) 3 - 1 - 4 - 2.
c) 1 - 2 - 3 - 4.
d) 3 - 4 - 1 - 2.
e) 4 - 1 - 3 - 2.
Alternative b) 3 - 1 - 4 - 2.
2. (Uerj) Isomery is the phenomenon that is characterized by the fact that the same molecular formula represents different structures.
Considering the flat structural isomerism for the molecular formula C4H8, we can identify isomers of the following types:
a) chain and position
b) chain and function
c) function and compensation
d) position and compensation
Alternative a) string and position
3. (OSEC) Propanone and isopropenol exemplify a case of Isomerism:
a) of metameria
b) of function
c) of tautomery
d) cis-tran
e) chain
Alternative c) of tautomery
See too: Exercises on Flat Isomerism