You aldehydes and the ketones they are very similar organic functions. Both have in their structure the carbonyl functional group (C = O), with the only difference that, in the case of aldehydes, it always appears at the end of the carbon chain, that is, one of the carbonyl carbon ligands is the hydrogen; ketones have the carbonyl between two other carbon atoms.
Aldehydes functional group:Functional group of ketones:
O O
║ ║
C C ─ H C C ─ C
For this reason, there are cases of functional isomerism between aldehydes and ketones. For example, below we present two functional isomers that have the same molecular formula (C3H6O), but one is an aldehyde (propanal) and the other is a ketone (propanone). See how this totally changes their properties and applications:
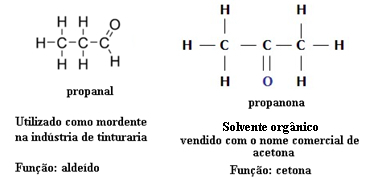
Example of function isomerism between aldehyde and ketone
Imagine that you are in a laboratory and you find a vial with a colorless liquid that has only the molecular formula C3H6O. What would you do to find out if it's a ketone or an aldehyde?
To solve problems like this, there are methods for differentiating aldehydes and ketones based on the reaction of these compounds against weak oxidizing agents. As shown below, when faced with weak oxidants, aldehydes react by being oxidized, while ketones do not react. We say thataldehydes act as reducing agents, but ketones do not, they only react as reducing agents in contact with energetic oxidants.
Aldehydes + Weak Oxidizers → carboxylic acid
O O
║ ║
C ─ C ─ H + [O] → C ─ C ─ OH
Ketones + Weak Oxidizers → Do not react
O
║
C ─ C ─ C + [O] → No reaction occurs
Based on that, it is enough then to carry out this reaction and see if the compound reacts or not. If it reacts, we know it's an aldehyde; if it doesn't react, it's a ketone.
In addition, the products formed in these aldehyde oxidation reactions are quite visible, with color changes occurring, as will be shown later.
There are three main methods of differentiating aldehydes and ketones, which are:
1- Tollens Reactive: This reagent is a ammonia solution of silver nitrate, ie it contains silver nitrate (AgNO3) and excess ammonium hydroxide (NH4OH):
AgNO3 + 3 NH4OH → Ag (NH3)OH + NH4AT THE3 + 2 H2O
Tollens Reactive (named after the German chemist Bernhard Tollens (1841-1918))
As explained in the text Making a Silver Mirror, when an aldehyde is brought into contact with Tollens reactive, it is oxidized to the corresponding carboxylic acid, while silver ions are reduced to Ag0 (metallic silver). If this reaction is carried out, for example, in a test tube, this metallic silver will deposit on the walls of the tube, resulting in the formation of a film called a silver mirror. This observed result is very beautiful and is used in the industrial mirror manufacturing process.
Do not stop now... There's more after the advertising ;)
The reaction that takes place can be represented as follows:
O O
║ ║
R ─ C ─ H + H2O → R ─ C ─ OH + 2e- + 2 H+
2 Ag+ + 2e- → 2 Ag0
2 NH3 + 2 H+ → 2 NH4+
O O
║║
R C ─ H + 2 Ag+ + 2 NH3 +H2O → R C ─ OH + 2 Ag0 + 2 NH4+
aldehyde Tollens reactivecarboxylic acid metallic silver (silver mirror)
On the other hand, if we put the ketone to react with the Tollens reactive, the formation of metallic silver will not occur, because the ketones cannot reduce the Ag ions.+.
2- Fehling Reactive: This reactive is a blue solution of copper sulphate II (CuSO4) in a basic medium, as it is mixed with another solution formed by sodium hydroxide (NaOH) and sodium and potassium tartrate (NaOOC-CHOH-CHOH-COOK). Tartrate is added to the copper II sulphate solution to stabilize it and prevent its precipitation.
CUSO4 + 2 NaOH → Na2ONLY4 + Cu(OH)2
Fehling Reactive (named after the German chemist Hermann von Fehling (1812-1885))
In contact with Fehling's reactive, an aldehyde forms the carboxylic acid by its oxidation, while copper ions (Cu)2+) present in the middle are reduced, forming a reddish-brown precipitate (more brick-like in color), which is cuprous oxide. Ketones, on the other hand, do not react - because they cannot reduce Cu ions2+.
O O
║ ║
R ─ C ─ H + 2 Cu(OH)2 → R ─ C ─ OH + Ass2O + 2 H2O
aldehyde reddish-brown precipitate
3- Benedict's Reactive: This reactive is also formed by a solution of copper II sulfate (Cu (OH)2) in a basic medium, but it is mixed with sodium citrate.
As with Fehling's reagent, in the case of the reaction between the aldehyde and Benedict's reagent, there are also copper ions (Cu2+) present in the medium that are reduced and form red cuprous oxide.
This reagent is widely used in tests to detect the presence and content of glucose in urine. Glucose has an aldehyde group in its structure, so it reacts with Benedict's reagent present in strips for these tests. From there, just compare the color of the ribbon with the color of the scale on the product's packaging.

Benedict's reagent is used to determine the glucose content in urine.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Differentiation of aldehydes and ketones"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/diferenciacao-aldeidos-cetonas.htm. Accessed on June 27, 2021.
Chemistry

Aldehydes, carbonyl compounds, carbonyl group, Main aldehydes, Ethanal, raw material in the pesticide and drug industry, Metanal, formaldehyde, plastic and resin industry.
Chemistry

Ketones, organic substances, carbonyl functional group, obtaining enamel solvent, propanone, ketone bodies in the bloodstream, extraction of oils and fats from plant seeds, solvents Organic.