Compensation isomerism or metamerism is a type of flat isomerism, that is, the difference between the isomers can be identified through the analysis of the flat structural formula of the molecule.
The word "metameria" comes from goal, which means "change", and mere, which means “parts”. In this type of isomerism, exactly that happens: a change of position in a part of the molecule, and this "part" is the heteroatom.
Heteroatom is any chemical element that appears in a carbon chain between two carbon atoms.
Thus, we have that, in compensating isomerism or metamerism, the isomers are differentiated by the location of the heteroatom in the chain.. This means that it is a special kind of position isomerism.
It is important to note that these isomers have the same functional group and the same chain type. (normal or branched, open or closed and will always be heterogeneous, due to the presence of the heteroatom).
Generally, the heteroatoms that appear attached to carbon in organic compounds are oxygen, nitrogen and sulfur. Therefore, metamerism normally occurs in ethers, esters, thioethers, amines (monosubstituted or disubstituted) and amides.
Do not stop now... There's more after the advertising ;)
See below for two examples of how this occurs with open and closed chain ethers:
- The two compounds below have the same function (ether), the same molecular formula (C4H10O) and the same type of chain (open, normal and heterogeneous), but they differ because oxygen is in different locations. In the first molecule, it is between carbons 1 and 2, and in the second, it appears between carbons 2 and 3:
H3C ─ O CH2 CH2 CH3 H3C CH2 ─ O CH2 CH3
methoxypropane ethoxyethane
This change seems small, but it completely changes the properties and applications of these compounds. For example, methoxypropane is used in industrial syntheses, while ethoxyethane is used as a common anesthetic (ether).
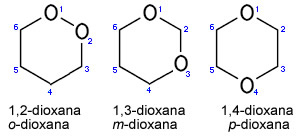
- Below are three dioxane metamers, note that they are all cyclic diethers with hexagonal chains, the only structural difference being the location of the heteroatoms.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Isomerism of offset or metamerism"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/isomeria-compensacao-ou-metameria.htm. Accessed on June 27, 2021.
Chemistry
Know what the various types of plane and spatial isomers are all about, such as function, position, chain, tautomerism, metamerism, cis-trans geometric and optical isomerism.