Hess' law was created by Swiss chemist Germain H. Hess (1802-1850), who is considered one of the founders of Thermochemistry. His law said:

That is, the ΔH depends only on the enthalpy values of the reagents and products, as in the expression:
| ΔH = ΔHreagents + ΔHproducts |
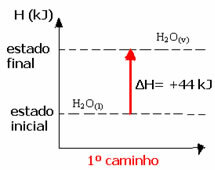
For example, consider the reaction where 1 mol of water (H2O) is transformed into water in the gaseous state. This reaction is performed twice; however, we chose different paths:
(1st) It was carried out in a single step:
H2O(1) → H2O(v) ΔH= +44 kJ
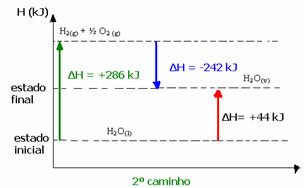
(2nd) It was carried out in two stages:
1st step: H2O(1) → H2(g) + ½ the2 (g) ΔH= +286 kJ
2nd stage: H2(g) + ½ the2 (g) → H2O(v) ΔH= -242 kJ
H2O(1) → H2O(v) ΔH= +44 kJ
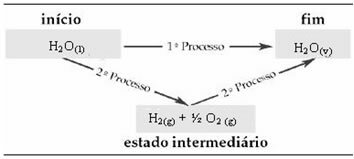
Note that regardless of whether one or two steps have been performed, the enthalpy change (ΔH) is always 44 kJ. This is because ΔH is the algebraic sum of the ΔH values of the steps that make up the process, that is, the intermediate processes:
| ΔH = ΔH1 + ΔH2 + ΔH3 + ... |
For example, in the second case, the total ΔH of the reaction was the sum of the ΔH of the first step (ΔH= +286 kJ) with that of the second (ΔH= -242 kJ). Thus, we obtained the same reaction value with a single step:
ΔH = (+286 + (-242)) kJ
ΔH = (+286 -242) kJ
ΔH = +44 kJ
Therefore, we are only interested in the initial and final values. In this case, the initial state corresponds to H2O(1) and the end to H2O(v).
This law has become very important in Thermochemistry, because certain chemical reactions cannot have their ΔH determined experimentally. However, according to Hess' Law the enthalpy of this type of reaction can be calculated from the enthalpies of other reactions (intermediate reactions).
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team