THE solubilitycan be defined as the maximum possible amount of a solute that can be dissolved in a certain amount of solvent at a given temperature.
This maximum amount that can be dissolved is also known as solubility coefficient or solubility degree. But the solubility of any substance depends, among other things, on the type of solvent in which the solute is dispersed.
For example, NaCl (sodium chloride - table salt) is very soluble in water, and in 1 L of water at 20ºC, we can solubilize up to 360 grams of this salt. But when the solvent changes to gasoline, under the same conditions of volume, temperature and pressure, the salt does not dissolve.
Why does the solubility of a substance vary so much from one solvent to another?
One of the factors is the polarity of the compounds involved. In the example cited, we have that the salt is polar, water is polar, and gasoline is non-polar. Salt is formed by atoms of sodium (Na) and chlorine (Cl) that bond through ionic bonds, in which sodium definitely donates an electron to chlorine, forming Na ions
+ and Cl-. As these ions have opposite charges, they attract and hold together (Na+Cl-).
This shows us that salt is really polar, every ionic bond is polar, as there is a difference in electrical charge in the compound.
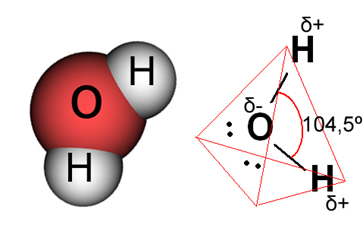
In the case of water, the existing bond is covalent, in which two hydrogen atoms share electrons with an oxygen atom. The water molecule has two dipoles, with oxygen having a partially negative charge and hydrogen having a partially positive charge (δ- O ─ H δ+). But, these dipoles do not cancel each other out, because the water molecule meets at an angle of 104.5º, showing that the distribution and charge along the molecule is not uniform. There is a greater negative charge density on the oxygen atom of the molecule. This shows us that the water molecule is really polar.
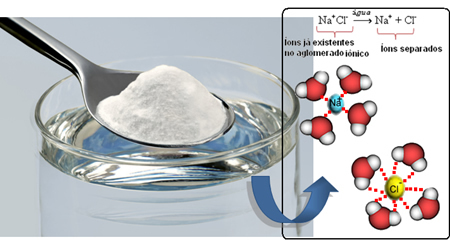
So when we mix the salt into the water, the positive part of the salt, which is the Na cations.+, is attracted by the negative part of water, which is oxygen, and the negative part of salt (Cl anions-) is attracted by the positive part of the water (H+). Consequently, the union Na+Cl- it is broken up, solubilizing the salt in the water.
Gasoline, on the other hand, is formed by a mixture of different hydrocarbons, which are non-polar, that is, the distribution of the electric charge of gasoline is uniform. Thus, there is no interaction of the salt ions with gasoline and it does not dissolve.
These and other similar cases lead us to the following conclusion:

However, this cannot be considered a general rule, as there are many cases of non-polar solutes that dissolve well in polar solvents and vice versa. So, to understand why this occurs, we have to consider yet another factor: the type of intermolecular force of the solvent and the solute.
Law about this in the text: "Relationship between Intermolecular Strength and Solubility of Substances”.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/relacao-entre-polaridade-solubilidade-das-substancias.htm

