Test your knowledge with easy, medium and hard questions about atomic models proposed by Dalton, Thomson, Rutherford and Niels Bohr.
Easy Level Questions
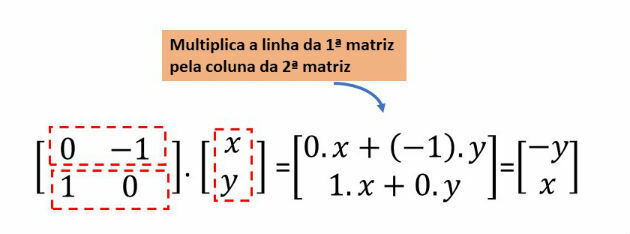
question 1
The image below represents which atomic model?

Answer: Rutherford-Bohr atomic model.
The Rutherford-Bohr atomic model was an improvement proposed by Bohr to the model created by Rutherford.
Rutherford's (1911) atom followed a planetary model, as if the nucleus were the Sun and the electrons corresponded to the planets.
In the Rutherford-Bohr model, electrons are in circular orbits with different energy levels and moving around the central nucleus.
question 2
Which scientist proposed the first modern atomic model that became known as the “billiard ball”?
a) Isaac Newton.
b) Democritus.
c) John Dalton.
d) Ernest Rutherford.
Correct alternative: c) John Dalton.
Dalton proposed around the 19th century that the atom was an indivisible particle, electrically neutral and extremely small.
For the scientist, all kinds of matter should be composed of atoms, which resembled a “billiard ball” because they are rigid and indivisible spheres.
Learn more aboutDalton's atomic model.
question 3
Check the incorrect alternative:
a) The first ideas concerning the internal structure of atoms were from Thomson.
b) In the Rutherford-Bohr atomic model, the electrons that rotate around the nucleus do not rotate randomly, but describe certain orbits.
c) Dalton's atomic model considered the existence of charges on atoms.
d) Democritus and Leucippus were the first to define the concept of matter and atom.
Incorrect alternative: c) Dalton's atomic model considered the existence of charges on atoms.
For Dalton, the atom was a massive, indivisible particle that could neither be created nor destroyed.
According to his atomic model, the atom would be the smallest particle of matter and cannot be subdivided, for example, into smaller units, such as electrons.
question 4
About the Rutherford model, consider the following statements as true or false:
a) Rutherford's Atomic Model suggests that the atom has the appearance of a planetary system.
b) Rutherford's Atomic Model became known as “plum pudding model” or “pudding with raisins” due to its appearance.
c) In Rutherford's Atomic Model, electrons revolve around the nucleus (made up of protons and neutrons), similarly to the planets that revolve around the Sun.
d) The Rutherford Atomic Model is also called the "Rutherford-Bohr Atomic Model"
Answer: V, F, V, F.
the truth. According to the atomic model proposed by Rutherford, the atom would be composed of a positively charged nucleus and the negatively charged electrons would be around it, as do the planets around the Sun.
b) FALSE. This name was given to the atomic model proposed by Thomson. For him, the atom would be a positively charged sphere with electrons, whose charge is negative, embedded in its surface.
c) TRUE. Rutherford presented his atomic model with an atom full of empty spaces. The central region would be positively charged and the region around the nucleus would be filled with electrons, much lighter than the nucleus' protons.
d) FALSE. Bohr proposed an improvement to Rutherford's model. For him, electrons would be at different energy levels.
Learn more aboutRutherford atomic model.
question 5
Atomic models describe some structural aspects of atoms. About this statement we can say that:
a) The atomic models were developed by the Greek scientists Leucippus and Democritus.
b) The main atomic models are: Rutherford Model and the Rutherford-Bohr Model.
c) The first atomic model developed was the Rutherford Atomic Model.
d) Atomic models were developed by scientists in order to better understand the atom and its composition.
Correct alternative: d) Atomic models were developed by scientists in order to better understand the atom and its composition.
A model is created to explain a phenomenon or experiment, taking into account the existing knowledge bases.
From the moment that new information emerged, through scientific discoveries, atomic models evolved so that there were no conflicts over the composition of matter.
Learn more aboutatomic models.
mid level questions
question 6
(UFJF-MG) Associate the statements with their respective guardians:
I - The atom is not indivisible and matter has electrical properties (1897).
II - The atom is a massive sphere (1808).
III - The atom is formed by two regions called the nucleus and the electrosphere (1911).
a) I - Dalton, II - Rutherford, III - Thomson.
b) I - Thomson, II - Dalton, III - Rutherford.
c) I - Dalton, II - Thomson, III - Rutherford.
d) I - Rutherford, II - Thomson, III - Dalton.
e) I - Thomson, II - Rutherford, III - Dalton.
Correct alternative: b) I - Thomson, II - Dalton, III - Rutherford.
I - Thomson. Cathode ray experiments led Thomson to discover that electrons were part of matter. Also, knowledge about radioactivity made him realize that the atom is neither massive nor indivisible.
II - Dalton. According to his model, the atom was a massive, indivisible sphere. So small, the number of atoms in matter could not be counted.
III - Rutherford. His studies on radioactive emissions led him to confirm the existence of the nucleus (positively charged region) and the electrosphere (region formed by electrons) according to the deviations observed when bombing a blade of gold.
Learn more aboutatom.
question 7
(UFRGS) Consider the following statements regarding the Rutherford experiment and the Rutherford-Bohr atomic model.
I - Most of the volume of the atom is constituted by the dense and positive nucleus.
II - Electrons move in stationary orbits around the nucleus.
III- The electron, when jumping from an outer to an inner orbit, emits a well-defined amount of energy.
Which ones are correct?
a) Only I.
b) Only II.
c) Only III.
d) Only II and III.
e) I, II and III.
Correct alternative: d) Only II and III.
I. WRONG. Most of the atom's volume is constituted by the electrosphere, the region of the atom where the electrons are located.
II. CORRECT In the Rutherford-Bohr model, electrons locate in orbits with specific energy levels around the nucleus.
III. CORRECT An atom in the ground state has its electrons located at the respective energy levels. If the electron changes from a higher energy level to a lower energy level, radiant energy is emitted.
Learn more aboutatomic structure.
question 8
(Vunesp-adapted) In 1913, Niels Bohr (1885-1962) proposed a model that provided an explanation for the origin of atomic spectra. In this model, Bohr introduced a series of postulates, among which, the energy of the electron can only assume certain discrete values, occupying allowed energy levels around the atomic nucleus. Considering the Bohr model, the different atomic spectra can be explained in terms of
a) the receipt of electrons by different elements.
b) the loss of electrons by different elements.
c) of the different electronic transitions, which vary from element to element.
d) the promotion of different electrons to more energetic levels.
e) the nuclear instability of different elements.
Correct alternative: c) of the different electronic transitions, which vary from element to element.
Bohr relied on three studies to create his atomic model. Are they:
- Rutherford Atomic Model
- Planck's quantum energy theory
- Line spectrum of chemical elements
For Bohr, the different atomic spectra vary from one element to another because electrons move around the nucleus in stationary orbits when the atom is in its state fundamental.
However, when jumping from one orbit to another, a certain amount of energy is released in the form of a quantum and, therefore, there are different electronic transitions.
Learn more about Bohr atomic model.
question 9
(PUC-RS) The historical acceptance of the idea that matter is composed of atoms was slow and gradual. In ancient Greece, Leucippus and Democritus are remembered for introducing the concept of the atom, but their proposals were rejected by other philosophers and fell into oblivion. In the late 18th and early 19th centuries, when Lavoisier's ideas were gaining widespread acceptance, the first modern atomic theory, proposed by _______, emerged. This theory postulated that the elements were made up of a single type of atom, while the composite substances were combinations of different atoms in certain proportions. Almost a hundred years later, studies with cathode rays led J. J. Thomson to the discovery of _______, a particle of very small mass and electrical charge _______, present in all known materials. A few years later, through experiments in which a thin gold leaf was bombarded with alpha particles, Rutherford came to the conclusion that the atom has at its center a small but massive _______ considerable.
The words that fill in the blanks correctly and respectively are gathered in
a) Dalton - electron - negative - nucleus
b) Bohr – cation – positive – electron
c) Dalton - neutron - neutral - proton
d) Bohr – photon – negative – anion
e) Dalton – proton – positive – nucleus
Correct alternative: a) Dalton – electron – negative – nucleus.
Dalton: postulated that the elements were made up of a single type of atom, while the composite substances were combinations of different atoms according to certain proportions.
Electron: It was discovered by Thomson when studying the electrical nature of matter, measuring the charge and mass of electrons, whose charge is negative.
Nucleus: discovered by Rutherford when bombing a gold blade and observing the deviations of radioactive emissions, since its charge is positive.
Learn more aboutelectrons.
question 10
(ESPM-SP) Rutherford's atom (1911) was compared to the planetary system (the atomic nucleus represents the sun and the electrosphere, the planets):
Electrosphere is the region of the atom that:
a) contains the negatively charged particles.
b) contains the positively charged particles.
c) contains neutrons.
d) concentrates practically the entire mass of the atom.
e) contains protons and neutrons.
Correct alternative: a) contains the negatively charged particles.
For Rutherford, the central region of the atom would be composed of a positive charge and around it would be the largest region of the atom, the electrosphere, whose electrons are distributed like the planets around of the sun.
Learn more aboutprotons.
Difficult Level Questions
question 11
(Udesc) Considering the most relevant atomic models, from a historical and scientific perspective, tick the correct alternative.
a) Until the discovery of radioactivity, the atom was considered indivisible (Dalton). The model that followed it was that of Thomson, who proposed the atom to be formed by a positively charged mass with electrons distributed in it.
b) In Dalton's model, the atom consisted of a positively charged nucleus and an electrosphere. The next model was that of Bohr who introduced the idea that electrons occupy orbitals with defined energies, this model is similar to the model of the solar system.
c) In Dalton's atomic model, the atom was considered indivisible. The successor model was Rutherford's, in which the atom consisted of a negatively charged nucleus and an electrosphere.
d) Dalton's model proposed that the atom was formed by a positively charged mass with electrons distributed in it. The next model was Rutherford's, in which the atom consisted of a positively charged nucleus and an electrosphere.
e) In Dalton's atomic model, electrons occupy orbitals with defined energies, this model resembles that of the solar system. The model that followed was Thomson's, which proposed the atom to be formed by a positively charged mass with electrons distributed in it.
Correct alternative: a) Until the discovery of radioactivity, the atom was considered indivisible (Dalton). The model that followed it was that of Thomson, who proposed the atom to be formed by a positively charged mass with electrons distributed in it.
While Dalton believed in the indivisibility of the atom, Thomson studied the electrical nature of matter and, with this, proved its divisibility by the existence of electrons (negative charge) around a sphere (charge positive).
Learn more aboutThomson atomic model.
question 12
(FAME) The model proposed by Bohr introduced a single quantum number to describe the behavior of the electron in the atom. The quantum mechanics model uses three quantum numbers.
Regarding the quantum numbers proposed in the Bohr model and in the quantum mechanics model, it is CORRECT to state that
a) the Bohr atomic model is related to a quantum number that describes about the orientation of orbitals.
b) the azimuthal quantum number has positive integer values and as this quantum number increases, the orbital becomes larger.
c) the level with the main quantum number n will consist of n sublevels, and each sublevel corresponds to a different allowable value of the secondary quantum number between 1 and n-1.
d) the relative energies of the electron in the hydrogen atom's orbitals have different values when the electron is in the orbitals of the same sublevel.
Correct alternative: c) the level with the main quantum number n will consist of n sublevels, and each sublevel corresponds to a different allowable value of the secondary quantum number between 1 and n-1.
The quantum mechanics model is the most modern and complex to describe the atom. Quantum numbers are used to indicate the location of electrons in orbitals.
The principal quantum number (n) indicates the energy level that the electron is at. The secondary or azimuthal quantum number (l) indicates the sublevel that the electron can be.
Learn more about quantum numbers.
question 13
(UFAL) One of the experiments conducted by Rutherford's team revolutionized the way physicists at the time began to imagine the atom. It consisted in the bombardment of thin gold blades to study the deflections (shifts) of alpha particles. According to the atomic model proposed by Rutherford, given the following statements
I. The atomic nucleus is extremely small relative to the size of the atom and it is in the nucleus where protons and neutrons are found.
II. The atom is a positively charged sphere in which negatively charged electrons would be embedded.
III. Matter is made up of atoms that are indivisible and indestructible particles.
IV. The atom is made up of two distinct regions: a dense nucleus, very small, and a region of very large volume, occupied by electrons, the electrosphere.
it turns out that they are correct
a) I, II, III and IV.
b) II and IV only.
c) II and III only.
d) I, III and IV only.
e) I and IV only.
Correct alternative: e) I and IV only.
I. TRUE. As the atom is composed of the nucleus (protons + neutrons) and the electrosphere (electrons), the atomic nucleus is extremely small in relation to the size of the atom.
II. FALSE. This model corresponds to the one proposed by Thomson. For Rutherford, the atom would be like a planetary system.
III. FALSE. His experiments showed that matter had different charges and empty spaces.
IV. TRUE. Making a comparison with the solar system, for Rutherford the nucleus would be like the Sun and the electrosphere would correspond to the planets.
Learn more aboutneutrons.
question 14
(Udesc) Electricity (from the Greek electron, meaning amber) is a physical phenomenon caused by electrical charges. There are two types of electrical charges: positive and negative. Charges with the same names (same sign) repel each other and those with different names (different signs) attract. According to the information, tick the correct alternative.
a) The phenomenon described above cannot be explained using the Dalton atomic model.
b) The phenomenon described above cannot be explained using the Thomson atomic model.
c) Protons have a negative electrical charge.
d) The phenomenon described above cannot be explained using the Rutherford atomic model.
e) Electrons have a positive electrical charge.
Correct alternative: a) The phenomenon described above cannot be explained using the Dalton atomic model.
For Dalton, the atom was an indivisible particle and therefore could not be divided into charges.
question 15
(PUC-RS) John Dalton was responsible for introducing the atomic theory into science in the early years of the nineteenth century. At that time, it was still not possible to know how many atoms of each element were included in the composition of simple molecules. Today we know that the water molecule formula is H2O and that ammonia is NH3. Dalton assumed that the simplest molecules were 1:1 combinations; thus, water would be HO and ammonia would be NH. Dalton introduced an atomic mass scale based on hydrogen, which had mass 1.
In Dalton's time, it was believed that, by mass, water had 1/8 hydrogen, and ammonia had 1/6 hydrogen. Thus, it was possible to conclude that the atomic masses of oxygen and nitrogen were respectively worth
a) 7 and 5.
b) 8 and 6.
c) 9 and 7.
d) 16 and 14.
e) 32 and 28.
Correct alternative: a) 7 and 5.
Water and ammonia are substances formed by the joining of elements.
If in water the amount of hydrogen represented 1/8, then of the eight parts into which it was divided, 7 corresponded to oxygen, being its contribution to the formation of the 7/8 molecule.
In ammonia, the amount of hydrogen represented 1/6, that is, dividing the molecule into 6 parts, only one represented hydrogen and the other 5 parts corresponded to nitrogen.
Read about the Evolution of Atomic Models.
Check out more issues with commented resolution in Exercises on Atoms.