The Enem Sciences and its Technologies test is composed of 45 objective questions multiple choice, worth a total of 100 points. In it, specific knowledge of Biology, Physics and Chemistry.
See below a list and a short summary of the subjects that involve the different contents that fall the most in the Natural Sciences and its Technologies test.
Biology
Molecules, Cells and Tissues
- Cell: Smallest unity of living beings with defined forms and functions.
- cell theory: Claims that all living beings are formed by cells.
- Cell organelles: They are like small organs that carry out essential activities for cells.
- Cell core: Where the genetic material (DNA) of organisms is found and is present in eukaryotic cells.
- cell division: Process by which a mother cell gives rise to daughter cells.
- Metabolism: Set of chemical reactions that take place in the cell and allow it to stay alive, grow and divide.
- Protein synthesis: Mechanism of protein production.
- Histology: Study biological tissues analyzing their structure, origin and differentiation.
- Cytology: The branch of biology that studies cells and their structures.
- Biotechnology: The use of technologies to create or modify living organisms.
heredity and diversity of life
- Heredity: Biological mechanism where the characteristics of each living being are transmitted from one generation to another.
- Genes and Chromosomes: Genes are tiny structures made up of DNA. In turn, the set of these structures form the chromosomes.
- Mendel's Laws: They are a set of fundamentals that explain the mechanism of hereditary transmission over generations.
- Introduction to genetics: Basic concepts in the field of biology that studies the mechanisms of heredity or biological inheritance.
- Genetic variability: Refers to variations in genes between individuals in a population.
- genetic engineering: Techniques of manipulation and recombination of genes that reformulate, reconstitute, reproduce and even create living beings.
- blood types: The most important are the ABO System and the Rh Factor.
- ABO System and Rh Factor: The ABO system classifies human blood into the four existing types: A, B, AB and O. The Rh Factor, on the other hand, is a group of antigens that determines whether the blood has a positive or negative Rh.
identity of living beings
- classification of living beings: System that organizes living beings into categories according to their common characteristics and evolutionary kinship relationships.
- Virus: They are infectious agents, microscopic and acellular (they do not have cells).
- prokaryotic cells: They do not have nuclear membrane or membranous structures inside.
- eukaryotic cells: Consists of plasma membrane, cytoplasm and nucleus.
- Autotrophs and Heterotrophs: Autotrophs are living beings that obtain nutrients and energy, taking advantage of sunlight, through photosynthesis, while heterotrophs obtain nutrients and energy, consuming other living beings.
- Phylogeny: It is the genealogical history of a species and its hypothetical relationships between ancestors and descendants.
- Embryology: Study all stages of embryonic development from fertilization, zygote formation until all organs of the new being are completely formed.
- Human anatomy: Study body structures, how they form and how they work together in the body (systems).
- Physiology: Study of the multiple chemical, physical and biological functions that guarantee the proper functioning of organisms.
Ecology and environmental sciences
- ecosystem: Set formed by biotic communities and abiotic factors that interact in a given region
- Brazilian ecosystems: The main Brazilian ecosystems are: Amazon, Caatinga, Cerrado, Atlantic Forest, Mata dos Cocais, Pantanal, Araucaria forest, Mangue and Pampas.
- Biotic and abiotic factors: The physical and chemical elements of the environment (abiotic factors) determine, to a large extent, the structure and functioning of living communities (biotic factors).
- Habitat and ecological niche: The habitat is where an animal lives and the niche is how it lives there.
- food web: Set of food chains linked in an ecosystem.
- Food chain: Corresponds to the feeding relationship, that is, the absorption of nutrients and energy between living beings.
- ecological pyramids: These are graphical representations of trophic interactions between species in a community.
- Biogeochemical cycles: Represent the movement of chemical elements between living beings and the planet's atmosphere, lithosphere and hydrosphere.
- Biomes of the WorldAttractions: There are seven main ones: Tundra, Taiga, Temperate Forest, Tropical Forest, Savannas, Prairie and Desert.
- Brazilian Biomes: There are six: Amazon, Cerrado, Caatinga, Atlantic Forest, Pantanal and Pampa.
- Natural resources: These are the elements offered by nature, which are used by man for his survival.
- Climate changes: Are climate changes across the planet.
- greenhouse effect and global warming: The greenhouse effect is a natural process that is intensified by human action and causes global warming.
Origin and evolution of life
- Origin of life: Explained by various theories developed in the search for answers.
- Abiogenesis and Biogenesis: Two theories formulated to explain the origin of life on Earth.
- What is universe?: Corresponds to the set of all existing matter and energy.
- Big Bang Theory: Holds that the Universe emerged from the explosion of a single particle - the primordial atom - causing a cosmic cataclysm.
- Evolution: Corresponds to the process of modification and adaptation of species over time.
- Human evolution: Corresponds to the process of change that gave rise to human beings and differentiated them as a species.
- Evolution theory: Current species are descended from other species that have undergone changes over time and transmitted new characteristics to their descendants.
- Darwinism: It is the set of studies and theories related to the evolution of species, developed by the English naturalist Charles Darwin.
- Neo-Darwinism: It is the modern theory of evolution that is based on the evolutionary studies of Charles Darwin, together with the discoveries of genetics.
- Natural selection: Occurs due to the need for survival and adaptation of species to the environment.
Quality of life of human populations
- Human Development Index (HDI): Evaluation of the development of humanity based on information about the quality of life and the economy of a territory.
- Social inequality: Social problem in which there is disproportionality in the standard of living of the inhabitants.
- Gross Domestic Product (GDP): Way to measure the production within a certain period of time.
- STD - Sexually Transmitted Diseases: These are diseases that can be transmitted from one person to another through sexual contact.
- drugs: These are substances that modify the body's functions, as well as people's behavior
- Teenage pregnancy: Pregnancy that occurs between 10 and 19 years is considered, according to the WHO.
- Brazil's social problems: The main ones are: unemployment, health, education, housing, violence and pollution.
- The importance of physical activity for health: Improves quality of life and, combined with a balanced diet, results in a healthy body, preventing diseases.
- Healthy eating: Consumption of foods with variety, moderation and balance.
Biology issues that fell in Enem
1. (Enem/2016) The proteins of a eukaryotic cell have signal peptides, which are sequences of amino acids responsible for their addressing to the different organelles, according to their functions. A researcher has developed a nanoparticle capable of carrying proteins into specific cell types. Now he wants to know if a nanoparticle loaded with a Krebs cycle blocking protein in vitro it is able to exert its activity in a cancer cell, being able to cut the energy supply and destroy these cells.
When choosing this blocking protein to load the nanoparticles, the researcher must take into account an addressing signal peptide to which organelle?
a) Nucleus.
b) Mitochondria.
c) Peroxisome.
d) Golgiense complex.
e) Endoplasmic reticulum.
Correct alternative: b) Mitochondria.
Obtaining energy occurs by breaking the bonds of molecules.
Through aerobic respiration, that is, in the presence of oxygen, glucose has its bonds broken in three stages:
- Glycolysis
- Krebs Cycle
- Oxidative Phosphorylation
The first step takes place in the cytosol, while the other two steps take place in the mitochondria.
Thus, the mitochondria have the function of carrying out cellular respiration, which produces most of the energy used in cellular functions.
The signal peptide must be destined to the mitochondria, because by blocking the Krebs Cycle, it is possible to cut the energy supply and destroy the cells.
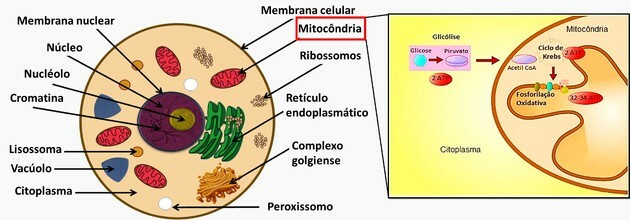
The cytoplasm is a bulky region that contains the nucleus and cell organelles.
The nucleus contains the genetic material (DNA and RNA).
Organelles function like organs in cells and each one performs a specific function.
The functions of the other organelles present in the question alternatives are:
- Endoplasmic reticulum: the smooth endoplasmic reticulum's function is to produce lipids that will compose the cell membranes, while the rough endoplasmic reticulum has the function to carry out the synthesis protein.
- The golgi complex: the main functions of the golgi complex are to modify, store and export proteins synthesized in the rough endoplasmic reticulum.
- Peroxisomes: the function is to oxidize fatty acids for cholesterol synthesis and cellular respiration.
2. (Enem/2017) The gray dolphins (Sotalia guianensis), mammals of the dolphin family, are excellent indicators of pollution in the areas in which they live, as they spend their entire lives - around 30 years - in the same region. In addition, the species accumulates more contaminants in its body, such as mercury, than other animals in its food chain.
MARCOLINO, B. Sentinels of the sea. Available in: http://cienciahoje.uol.com.br. Accessed on: 1 Aug. 2012 (adapted).
Gray dolphins accumulate a higher concentration of these substances because:
a) are herbivorous animals.
b) are detritivorous animals.
c) are large animals.
d) digest food slowly.
e) are at the top of the food chain.
Correct alternative: e) are at the top of the food chain.
It is possible to find out about the ecosystem where the gray dolphins live because these animals spend their lives in the same region. Therefore, any changes that can be observed in these animals come from changes in the place where they live.
In a food chain, one being becomes another's food, demonstrating the interactions of species in a location.
The components of a food chain are inserted at trophic levels, which correspond to the order in which nutrients are absorbed and energy is obtained between living beings.
In the ecosystem in which the dolphin lives, it is inserted at the top of the food chain.
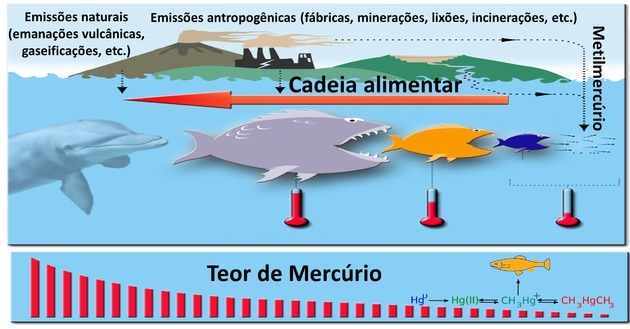
When the dolphin feeds, the animals present in the previous trophic levels have already absorbed several other organisms.
Heavy metals such as mercury are not biodegradable and are present in industrial activities, volcanoes, electronic waste and mines.
Bioaccumulation occurs when these toxic substances progressively accumulate in trophic levels. Thus, the highest mercury content will be found at the most distant trophic levels.
The concentration of this metal will be higher in the dolphin predator than in its prey, for example fish, shrimp and squid.
Although they are large animals, this does not justify bioaccumulation, and slow digestion does not interfere, as mercury is not biodegradable.
Herbivorous animals consume autotrophic beings such as algae, while detritivores feed on organic waste.
See too:Biology in Enem.
3. (Enem/2017) The Atlantic Forest is characterized by a great diversity of epiphytes, such as bromeliads. These plants are adapted to this ecosystem and are able to capture light, water and nutrients even living on trees.
Available at: www.ib.usp.br. Accessed on: Feb 23 2013 (adapted).
These species capture water from (a)
a) organism of neighboring plants.
b) soil through its long roots.
c) rain accumulated between its leaves.
d) crude sap of host plants.
e) community that lives in its interior.
Correct alternative: c) rain accumulated between its leaves.
Ecological relationships demonstrate the relationships between living beings and the environment in which they live, determining how they survive and reproduce.
Epiphytism is a harmonious ecological relationship between two species, where a species such as the bromeliad uses the trees to obtain shelter, without harming it.
Because they have different sizes, bromeliads find protection on the surfaces of larger trees, fixing their roots on the host tree.
The shape of the leaves enables the accumulation of rainwater and the micro scales promote the absorption of water and nutrients.
The roots of bromeliads are used only to attach themselves to the plants, thus establishing a relationship of tenants in which the epiphyte benefits, but does not harm the tree.
For more commented questions about Biology on Enem, we have prepared this list: Biology Issues in Enem.
Physics
energy, work and power
- work in physics: Energy transfer due to the action of a force.
- Energy: Represents the ability to produce work.
- Types of energy: Mechanical, thermal, electrical, chemical and nuclear.
- Kinetic energy: Energy associated with the movement of bodies.
- Potential energy: Energy related to the position of bodies.
- Strength: Action exerted on a body capable of modifying the state of rest or changing the amount of movement.
- Electric power: How quickly a job is done.
- Electric potential: Work of the electrical force on an electrified charge when moving between a point in relation to a reference point.
- Physics formulas: Relationships between quantities involved in the same physical phenomenon.
Mechanics, Motion Studies and Newton's Law Applications
- Quantity of movement: Vector quantity defined as the product of the mass of a body and its velocity.
- uniform movement: Represents the displacement of a body from a given reference frame, at constant speed.
- evenly varied movement: Velocity is constant over time and is nonzero.
- Uniform rectilinear movement: The body is under constant velocity, however, the trajectory taken by the body is in a straight line.
- Uniformly varied rectilinear movement: It is performed in a straight line and has a variation of speed always in the same time intervals.
- Newton's Laws: Fundamental principles used to analyze the movement of bodies.
- Gravity: Fundamental force that regulates objects at rest.
- Inertia: Property of matter that indicates resistance to change.
Wave phenomena and waves
- waves: Disturbances that propagate through space without transporting matter, only energy.
- mechanical waves: Disturbances that transport kinetic and potential energy through a material medium.
- Electromagnetic waves: Results from the release of electrical and magnetic energy sources together.
- Sound waves: These are vibrations that produce auditory sensations when they penetrate our ear.
- gravitational waves: They are ripples in the curvature of space-time that propagate through space.
Electrical and Magnetic Phenomena
- Electricity: Area of Physics that studies the phenomena caused by the work of electrical charges.
- electrostatics: Study electrical charges without movement, ie in a state of rest.
- Electrodynamics: Study the dynamic aspect of electricity, that is, the constant movement of electrical charges.
- Electromagnetism: Studies the relationship between the forces of electricity and magnetism as a unique phenomenon.
- Electrification processes: Methods where a body is no longer electrically neutral and becomes positively or negatively charged.
- Ohm's Laws: Determine the electrical resistance of conductors.
- Kirchhoff's Laws: Determine the strengths of currents in electrical circuits that cannot be reduced to simple circuits.
Heat and thermal phenomena
- heat and temperature: Heat designates the exchange of energy between bodies, while temperature characterizes the agitation of molecules in a body.
- heat spread: Heat transmission that can occur through conduction, convection or radiation.
- thermometric scales: They are used to indicate temperature, that is, the kinetic energy associated with the movement of molecules.
- Calorimetry: Studies the phenomena related to thermal energy exchanges.
- specific heat: Physical quantity related to the amount of heat received and its thermal variation.
- sensible heat: Physical quantity that is related to the variation in the temperature of a body.
- latent heat: Physical quantity that designates the amount of heat received or given by a body while its physical state changes.
- thermal capacity: Quantity that corresponds to the amount of heat present in a body in relation to the temperature variation suffered by it.
- Thermodynamics: Area of Physics that studies energy transfers.
Optics, optical phenomena, light refraction
- Light: Electromagnetic wave sensitive to the naked eye.
- light refraction: Optical phenomenon that occurs when light undergoes a change in the propagation medium.
- light reflection: Optical phenomenon of the incidence of light on a reflecting surface, returning to its point of origin.
- Speed of light: Speed with which light travels in a vacuum and propagation in different media.
Hydrostatics
- Hydrostatics: Fluid characteristics such as hydrostatic pressure, density and buoyant force.
- hydrostatic pressure: Concept and formulas for calculating hydrostatic pressure and total pressure.
- Stevin's Theorem: Relation between atmospheric and liquid pressure variation.
- Archimedes' Theorem: Calculation of the resultant force exerted by the fluid on a given body (buoyancy theorem).
Physics issues that fell in Enem
1. (Enem/2017) Fuse is an overcurrent protection device in circuits. When the current flowing through this electrical component is greater than its maximum rated current, the fuse blows. In this way, it prevents the high current from damaging the circuit devices. Suppose the electrical circuit shown is powered by a voltage source U and that the fuse supports a rated current of 500 mA.
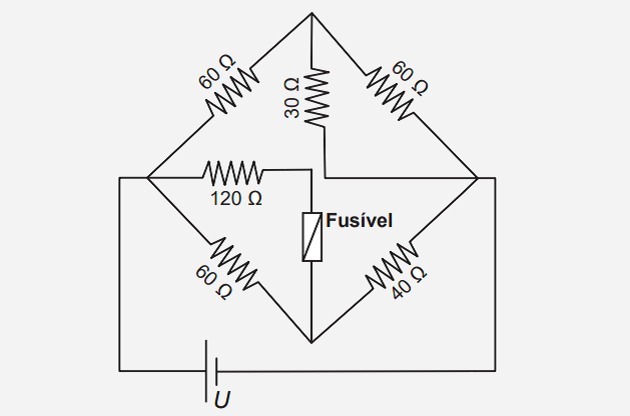
What is the maximum voltage value U so the fuse doesn't blow?
a) 20 V
b) 40 V
c) 60V
d) 120V
e) 185V
Correct alternative: d) 120 V
The circuit proposed in the question is formed by a mixed association of resistors. We also know that the maximum current supported by the fuse is 500 mA (0.5 A).
To find out the maximum value of the battery voltage, we can isolate the part of the circuit where the fuse is located, as shown in the figure below.
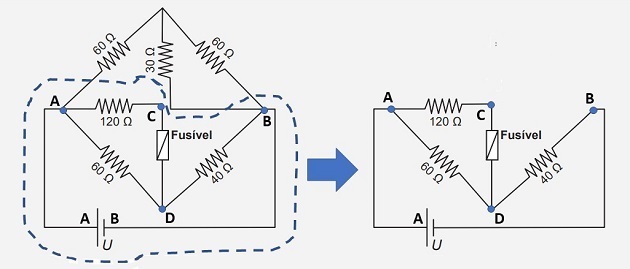
This is possible, since the “top” part of the circuit is subjected to the same voltage as the “bottom” part (highlighted part in the image), since its terminals are connected to the same points (A and B).
Let's start by finding the voltage value at the 120 resistor terminals. The current that passes through this resistor (i1) is the same one that goes through the fuse. Therefore, we have:
UB.C= 0.5,120 = 60V
This will be the same voltage as the 60 resistor terminals. are subjected, as it is connected in parallel with the 120 resistor.
.
Thus, we can find the current value (i2) that goes through this resistor:
The current i3 that crosses the 40 resistor is equal to the sum of i1 Hey2, i.e:
i3 = 1+0.5 = 1.5 A
Knowing this value we can calculate the voltage value at the resistor terminals of 40 :
Udb=1.5.40=60V
Thus, the circuit voltage will be equal to the sum of UB.C with Udb, that is:
U = 60 + 60 = 120 V
2. (Enem/2017) In some homes, electrified fences are used in order to keep out possible invaders. An electrified fence works with an electrical potential difference of approximately 10,000 V. In order not to be lethal, the current that can be transmitted through a person must not be greater than 0.01 A. The body electrical resistance between a person's hands and feet is around 1 000 1.
In order for the current not to be lethal to a person touching the electrified fence, the voltage generator must have an internal resistance that, in relation to that of the human body, is
a) practically null.
b) approximately equal.
c) thousands of times larger.
d) of the order of 10 times larger.
e) run 10 times smaller.
Correct alternative: c) thousands of times larger.
To resolve the issue we have to compare the generator's internal resistance with the human body's resistance. For this, we will use the following equations:
(generator equation)
U = R.i (Ohm's Law)
Note that r is the generator's internal resistance and R is equal to the body's resistance. Equating the two equations and substituting the values, we have:
Now, we need to find out how many times the generator's internal resistance must be greater than the body's resistance. For this, let's divide one by the other, that is:
Therefore, the generator's internal resistance should be around 1000 times greater than the person's body resistance.
3. (Enem/2017) A driver who answers a cell phone call is led to inattention, increasing the possibility of accidents occurring due to the increase in his reaction time. Consider two drivers, the first attentive and the second using a cell phone while driving. They accelerate their cars initially to 1.00 m/s2. In response to an emergency, they brake with a deceleration equal to 5.00 m/s2. The attentive driver applies the brake at a speed of 14.0 m/s, while the inattentive driver, in a similar situation, takes 1.00 second longer to start braking.
How far does the inattentive driver travel more than the attentive driver, until the cars come to a complete stop?
a) 2.90 m
b) 14.0 m
c) 14.5 m
d) 15.0 m
e) 17.4 m
Correct alternative: e) 17.4 m
Let's find the distance covered by the 1st driver by applying the Torricelli equation, that is:
v2 = v02 + Mondays
The initial speed of the first car is equal to 14 m/s, its final speed is equal to zero, because the car has stopped and its acceleration is equal to - 5 m/s2. Substituting these values in the equation, we have:
Now, let's look at the 2nd driver's situation. As he took 1 s longer before hitting the brake, the distance covered in that time interval can be found by applying the equation:
v = v0 + at
Considering that its acceleration was 1 m/s2 and that its initial speed was also 14 m/s, we found:
v = 14 + 1.1 ⇒ v2 = 15 m/s
To find the distance covered in this time interval, let's apply the Torricelli equation:
When applying the brake, its speed was equal to 15 m/s and its acceleration was equal to -5 m/s2. To find the distance traveled to stop, we will again use the Torricelli equation:
The total distance covered by the 2nd car will be equal to:
at2 = Δs' + Δs"
at2 = 14,5 + 22,5
at2 = 37.0 m
To find the distance that the inattentive driver traveled the most, just do:
37.0 - 19.6 = 17.4 m
See too:Physics in Enem.
Chemistry
chemical transformations
- chemical transformations: Actions that result in the formation of new substances
- Chemical bonds: Unions between atoms of the same or different elements.
- Chemical reactions: Rearrangement of atoms to form new substances.
- Chemical balance: Phenomenon that occurs in reversible chemical reactions, when the speed of the direct and inverse reactions are equal.
- Atomic models: They bring together the atomic models of Dalton, Thomson, Rutherford and Bohr.
- atomic structure: Composed of three fundamental particles: protons (with a positive charge), neutrons (neutral particles) and electrons (with a negative charge).
- Chemical elements: Fundamental element of matter composed of a group of atoms with the same atomic number.
- Periodic table: Classification of chemical elements in ascending order of atomic number.
- Stoichiometric calculations: Quantitative analysis of the composition of substances consumed and formed in a chemical reaction.
Materials, their properties and uses
- properties of matter: Physical or chemical characteristics that make up the materials.
- physical states of matter: Solid, liquid, gas, plasma and Bose-Einstein condensate.
- Physical state changes: Are condensation or liquefaction, solidification, fusion, vaporization and sublimation.
- ion, cation and anion: Ion is an electrically charged chemical species. A cation has a positive charge, an anion has a negative charge.
- intermolecular forces: Forces exerted to hold two or more molecules together.
- Molecule: It is a stable grouping of two or more identical or different atoms joined through covalent bonds.
- Molecular Geometry: It is the way that demonstrates how atoms arrange themselves in a molecule.
- molecular formula: It is the expression of chemical symbols and indices of the components of a molecule.
- structural formula: Represents how atoms bond together.
Water
- Water: One of the most important natural resources for humanity. It is made up of one oxygen atom and two hydrogen atoms.
- water properties: It is an excellent solvent because it is capable of dissolving a huge amount of substances.
- water density: It is 1 g/cm3 (it reads: one gram per cubic centimeter). This value corresponds to water at 25 °C.
- the importance of water: Water is the source of life on the planet. In this sense, when there is a lack of water, life is threatened.
- Physical states of water: It is found in nature in three physical states: liquid, solid and gas.
chemical solutions
- chemical solutions: They are homogeneous mixtures formed by two or more substances.
- Colloidal solutions: These are mixtures that present the appearance of a homogeneous mixture due to the size of the dispersed particles being between 1 and 100 nm.
- Solubility: It is the physical property of substances to dissolve, or not, in a given liquid.
- Concentration of solutions: Corresponds to the amount of solute present in a given amount of solvent.
- Dilution of solutions: It consists of adding solvent to a solution, without modifying the amount of solute.
Compounds and Chemical Substances
- Acids: These are substances that release positive hydrogen ions or protons in an aqueous solution.
- Bases: These are substances formed by the union of a cation and an anion, which release hydroxyl ions (OH anions–) in an aqueous solution.
- salts: Salts are the result of the reaction of an acid with a base.
- Oxides: These are binary ionic or molecular compounds, where oxygen molecules are linked to other elements.
Chemical Transformations and Energy
- thermochemistry: It is the part of chemistry that studies the involvement of the amount of heat (energy) in chemical reactions.
- Endothermic and exothermic reactions: Amount of heat absorbed or released during chemical reactions.
- enthalpy: It is the energy exchanged in the energy absorption and release reactions.
- Hess' Law: The enthalpy change (ΔH) in a chemical reaction depends only on the initial and final states of the reaction, regardless of the number of reactions.
- Electrochemistry: It is the area of Chemistry that studies the reactions that involve the transfer of electrons and the interconversion of chemical energy into electrical energy.
- Electrolysis: Non-spontaneous chemical reaction that involves an oxidation-reduction reaction, which is caused by an electrical current.
- Faraday's law: When there is variation in the magnetic flux through a circuit, an induced electromotive force will arise in it.
- Radioactivity: Nuclear phenomenon that results from the emission of energy by atoms, caused as a result of a disintegration or instability of chemical elements.
- Nuclear fission: It is the process of dividing the unstable atomic nucleus into other more stable nuclei.
- Nuclear fusion: It is the joining of atoms that have light nuclei. The joining of these atoms results in an atom with a heavier nucleus.
carbon compounds
- Organic chemistry: Branch of Chemistry that studies carbon compounds, which are those formed by carbon atoms.
- Organic functions: Classification of groups of organic compounds with similar characteristics.
- carbon chains: Structure of organic compounds according to the arrangement of atoms and bonds.
- Hydrocarbons: Compounds formed by carbon and hydrogen, with general formula CxHy.
- Inorganic chemistry: Branch of Chemistry that studies substances formed by chemical elements, except carbon.
- Inorganic Functions: Groups of inorganic compounds that have similar characteristics.
Relations of chemistry with technologies, society and the environment
- Pollution: Introduction of substances or energy accidentally or intentionally into the environment, with negative consequences for living beings.
- Types of pollution: The types depend on the affected resource and the type of waste generated, the main ones being: air, soil, water, thermal, sound, light, visual and radioactive.
- Ozone layer: It is an ozone gas blanket present in the stratosphere, which protects the planet from harmful ultraviolet radiation to living beings.
- industrial waste: It comes from processes developed in industries, that is, from the secondary sector.
Chemical energies in everyday life
- Petroleum: Natural substance composed of several organic components, especially hydrocarbons.
- Natural gas: It originates in the degradation of organic matter, in underground deposits mixed or not with oil. It is composed of a mixture of methane, in greater quantity, and other alkanes, in addition to CO2, H2Y and N2.
- Mineral coal: It is a non-renewable natural resource originated from plant remains over millions of years.
- Fossil fuels: These are non-renewable natural resources, originating from organic debris accumulated in the earth's crust over millions of years.
- biomass: All organic matter, of plant or animal origin, used in energy production.
- Biofuels: It is all material used to generate energy from organic biomass.
- Renewable energy: It is the energy obtained from sources that regenerate spontaneously or through the adequate intervention of man.
Chemistry issues that fell in Enem
1. (Enem/2016) In mid-2003, more than 20 people died in Brazil after ingesting a suspension of barium sulfate used as contrast in radiological examinations. Barium sulfate is a very poorly soluble solid that does not dissolve even in the presence of acids. The deaths occurred because a pharmaceutical laboratory supplied the product contaminated with barium carbonate, which is soluble in an acidic medium. A simple test to check for soluble barium ions could have averted the tragedy. This test consists of treating the sample with an aqueous solution of HCl and, after filtering to separate the insoluble barium compounds, an aqueous solution of H is added2ONLY4 on the filtrate and observed for 30 min.
TURBINO, M.; SIMONI, J.A. Reflecting on the Celobar® case. New Chemistry, no. 2, 2007 (adapted).
The presence of soluble barium ions in the sample is indicated by the
a) heat release.
b) color change to pink.
c) precipitation of a white solid.
d) formation of nitrogen gas.
e) chlorine gas volatilization.
Correct alternative: c) precipitation of a white solid.
Barium sulfate, used in exams as a contrast due to its low solubility, is excreted by the body. Barium carbonate has increased solubility in an acidic medium.
Our organism produces gastric juice to maintain stomach acidity and favor the action of enzymes in digestion.
The acid present in the body is hydrochloric acid, which increases the solubility of barium carbonate and consequently its death due to the absorption of barium ions.
1st step: determine the formulas of the compounds mentioned in the text.
| Compound | Cation | anion | Formula |
| Barium sulphate | Ba2+ | ONLY42- | BASO4 |
| barium carbonate | Ba2+ | CO32- | Spleen3 |
2nd step: double exchange reaction with HCl.
In this type of reaction, when two compounds react with each other, they exchange elements or radicals as follows:
At this stage, the only one that reacts with the acid is barium carbonate.
Carbonic acid is a weak and unstable acid formed by diluting carbon dioxide in water.
The double exchange reaction with hydrochloric acid then is:
Therefore, carbon dioxide may be released.
3rd step: double exchange reaction with H2ONLY4.
When carrying out the filtration, what is retained in the filter is the barium sulphate, which has not reacted, and the soluble barium chloride salt is filtered.
With the addition of sulfuric acid to the solution, the reaction occurs:
The initial solution indicates the presence of barium carbonate, as the test resulted in the formation of barium sulphate, a white precipitate.
See too:Chemistry in Enem.
2. (Enem/2017) A major turning point in the modern history of agriculture took place after World War II. After the war, governments had faced a huge surplus of ammonium nitrate, an ingredient used in the manufacture of explosives. From there, the ammunition factories were adapted to start producing fertilizers with nitrates as their main component.
SOUZA, F. THE. Natural/organic agriculture as a tool for biological fixation and maintenance of nitrogen in the soil: a sustainable CDM model. Available at: www.planetaorganico.com.br. Accessed on: July 17 2015 (adapted).
In the natural nitrogen cycle, the equivalent of the main component of these industrial fertilizers is produced in the step of
a) nitration.
b) nitrosation.
c) ammonification.
d) denitrification.
e) biological fixation of N2.
Correct alternative: a) nitration.
Nitrogen is a gas that is present in the air in large amounts.
The N2 atmospheric is very stable due to the triple bond that binds nitrogens and, therefore, it is not chemically reactive.
Nitrogen is very important for living beings, as it is part of biochemical compounds such as amino acids and nucleic acids, being acquired through food.
Bacteria present in the soil and in the roots of legumes are able to fix nitrogen through a cycle in which a flow of matter and energy occurs.
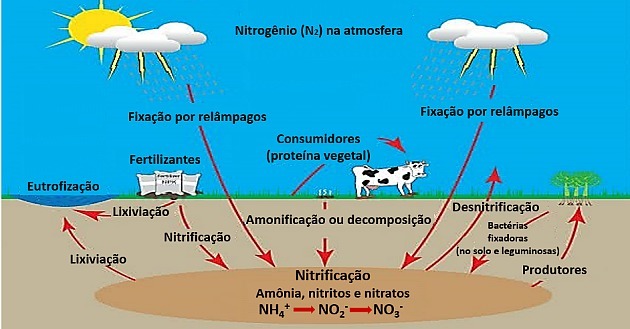
In the first step, the biological nitrogen fixation by bacteria occurs Rhizobium, turning it into ammonia.
Fixation also occurs by physical phenomena, such as lightning, producing small amounts of ammonia.
In ammonification, wastes from animal metabolism, such as urea, are transformed into ammonia by soil bacteria.
Nitrification transforms ammonia into nitrate through two steps:
First, nitrosation occurs, where bacteria nitromonas oxidize ammonia to nitrite.
Then in the nitration, by the action of bacteria Nitrobacter, nitrite is converted to nitrate also through oxidation.
The nitrate is then assimilated by most plants.
Therefore, industries have adapted the use of nitrate for applications such as fertilizers.
Excess nitrate is transformed by pseudonomas in nitrogen gas and returns to the atmosphere in the denitrification step.
3. (Enem/2017) A common fact when cooking rice is the pouring of part of the cooking water on the blue flame of the fire, changing it to a yellow flame. This color change can give rise to different interpretations, related to the substances present in the cooking water. In addition to table salt (NaCl), it contains carbohydrates, proteins and mineral salts.
Scientifically, it is known that this change in the color of the flame occurs by the
a) reaction of cooking gas with salt, volatilizing chlorine gas.
b) photon emission by sodium, excited by the flame.
c) production of yellow derivative, by reaction with carbohydrate.
d) reaction of the cooking gas with water, forming hydrogen gas.
e) excitation of protein molecules, with formation of yellow light.
Correct alternative: b) emission of photons by sodium, excited by the flame.
When salt is in contact with water, ionic dissociation occurs as follows:
And sodium and chlorine ions are solvated by water molecules.
When part of the cooking water is spilled, the sodium ions come into contact with the energy produced in the flame and what happens next is explained by the Rutherford-Bohr atomic model:
When receiving energy, the electrons are excited to an outer layer, ie, more energetic. Upon returning to a less energetic state, there is the release of energy in the form of well-defined color or electromagnetic radiation, the photons.
This movement is known as a quantum leap, that is, an atomic electronic transition occurs.
For more questions about Chemistry on Enem, we have prepared this list: Chemistry Questions in Enem.
Quiz Enem: Science of Nature
Also read about:
- Enem questions
- Simulated Enem (questions commented by experts)
- Human Sciences and its Technologies
- Languages, Codes and Their Technologies
- Subjects that most fall in Enem

