The melting point and boiling point represent the temperature at which a substance changes state at a given pressure.
In the case of the melting point, the substance changes from the solid state to the liquid state. The boiling point refers to the change from a liquid to a gaseous state.
For example, ice begins to turn into water in liquid form when its temperature is equal to 0°C. Hence, the melting point of water is 0 °C (under a pressure of 1 atmosphere).
To change from liquid to steam, water must reach a temperature of 100°C. Thus, the boiling point of water is 100 °C (under a pressure of 1 atmosphere).

Fusion point
When a substance in solid state receives heat, there is an increase in the degree of agitation of its molecules. Consequently its temperature also increases.
Upon reaching a certain temperature (melting point), the agitation of the molecules is such that it breaks the internal bonds between atoms and molecules.
At this point, the substance begins to change its state and will go into a liquid state if it continues to receive heat.
During the Fusion its temperature remains constant, as the heat received is used solely for the change of state.

The heat per unit of mass required to change phases is called latent heat of fusion (Lf) and is a characteristic of the substance.
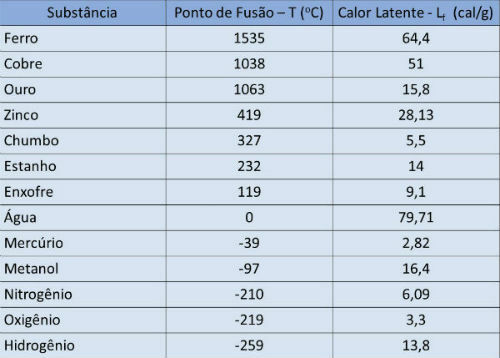
Melting point and latent heat table
In the table below we indicate the melting point temperature and the latent heat of some substances to atmospheric pressure.
Boiling point
THE boiling it is characterized by the rapid passage from a liquid to a gaseous state, with the formation of vapors (bubbles) inside the liquid.
As in fusion, there is a temperature (boiling point) at which a given substance changes from a liquid to a gaseous state.
For this to occur, the substance must receive heat. During the entire phase change, the temperature remains constant.
the latent heat of vaporization (Lv) is the amount of heat per unit of mass required for a substance to change phase.
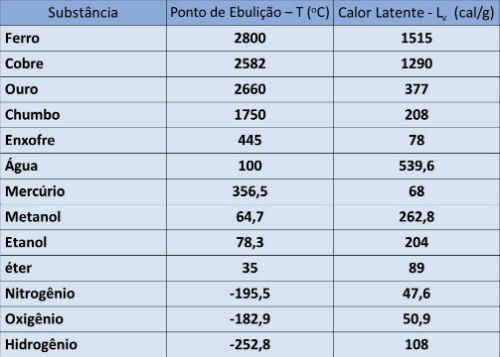
Boiling point and latent heat table
In the table below, we indicate the boiling point temperature and the latent heat of vaporization of some substances at atmospheric pressure.
Pressure interference
The temperature of the melting point and the boiling point depends on the pressure exerted on the substance.
In general, substances increase in volume when they undergo fusion. This fact means that the higher the pressure, the higher the temperature for the substance to change its phase.
The exception occurs with some substances, including water, which decreases its volume when melted. In this case, higher pressure will lower the melting point.

A decrease in pressure causes the boiling point of a given substance to be lower, meaning the substance will boil at a lower temperature.
For example, in places above sea level water boils at temperatures below 100°C. As a result, it takes much longer to cook in these places than in places at sea level.
Read too:
- Physical State Changes
- Thermometric Scales
- Thermometric Scales - Exercises
- Physical States of Water
- Phases diagram
- Solidification
- Condensation
- Colligative Properties
- Periodic Properties
- Evaporation
- Exercises on the Periodic Table