A neutralizing reaction occurs when an acid reacts with a base to form water and salt. The acid provides the H ions+ and the base provides the OH ions- for the formation of water (H2O):
1 hour+(here) + 1 OH-(here) → H2O(ℓ)
This type of reaction is called “neutralization” because the pH of the medium is neutralized; the pH of water is 7.0 (neutral).
For these reactions to occur it is necessary to release a certain amount of heat, as only a part of the energy of the ions is used to form the bonds that result in water molecules, while the rest of the energy is released to the quite. This released energy is called Neutralization Enthalpy (∆Hneutralization).

Do not stop now... There's more after the advertising ;)
Since it releases heat, it corresponds to exothermic reactions, in which the enthalpy (global energy of the system) will always be negative, less than zero.
In the case of a reaction between strong acids and strong bases, the neutralizing enthalpy value will always be equal to – 13.8 kcal/mol or – 57.7 kJ/mol. This happens because the bases and strong acids are completely dissociated in solution and, therefore, the only The reaction responsible for the manifestation of heat will be the formation of water, as shown in the three examples below:
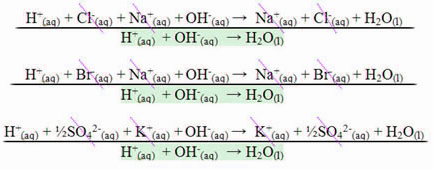
In the case of neutralization reactions involving weak acids or bases, the neutralizing enthalpy value will be less than –57.7 kJ/mol.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Enthalpy of Neutralization"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/entalpia-neutralizacao.htm. Accessed on June 28, 2021.