A reversible reaction that is in balance will only have its balance shifted if there is any external change, as a balance never shifts of its own accord.
One such change is concentration variation, which involves removing or adding reagents or products.
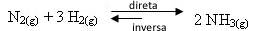
Let's look at an example:
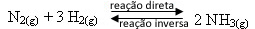
- Addition of reagents:
If we add more hydrogen gas or more nitrogen gas to the equilibrium, the concentrations of the reactants will increase and, with that, the number of effective shocks between their molecules will also rise, resulting in an increased rate of development of the direct ammonia formation reaction (NH3(g)).
This means that the addition of reagents shifts the balance to the right side, towards the formation of products:

This is in accordance with the Principle of Le Chatelier which says that when some disturbance is caused in a system in equilibrium, it moves in the direction of canceling this disturbance, trying to adjust to a new equilibrium.
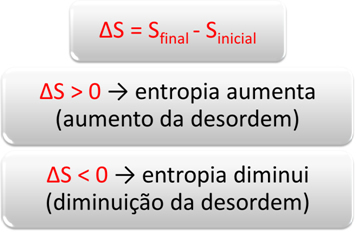
In the case above, it will happen that, over time, the amount of ammonia will increase, reaching an equilibrium again. Thus, the ratio between the concentrations of products and reagents will remain constant, that is, the value of the equilibrium constant Kc will remain the same.
Kc = __[NH3]2__↑
[N2]. [H2]3 ↑
This will also happen for the other cases, that is, no variation in the concentration of reagents or products will change the value of Kc.
- Addition of products:
If we add more ammonia, increasing its concentration, part of it will turn into gases nitrogen and hydrogen, increasing the rate of development of the inverse reaction of formation of the reagents.
Do not stop now... There's more after the advertising ;)
This means that the addition of products shifts the balance to the left, towards the formation of reagents:
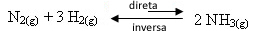
- Removal of reagents:
If we remove one or both of the reagents, their concentrations will decrease and, consequently, the rate of development of the direct reaction will decrease. Thus, the balance will be shifted towards the formation of more reactants, which is to the left:
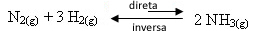
- Product withdrawal:
If we decrease the concentration of the products, the rate of the reverse reaction will decrease, increasing the rate of development of the forward reaction. This means that the balance will shift to the right:
Briefly, we can say the following:

It is important to emphasize that the variation in solids concentration does not shift equilibrium.
So, in the reaction below, if we remove or add CO2(g) or CO(g), there will be a shift in balance. But if we decrease or add C(s), nothing will happen with chemical balance:
Ç(s) + CO2(g) ↔ 2 CO(g)
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Variation of Concentration and Displacement of Chemical Equilibrium"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/variacao-concentracao-deslocamento-equilibrio-quimico.htm. Accessed on June 28, 2021.