You peptides are biomolecules formed by two or more amino acids. The binding of peptides occurs through covalent chemical bonds, called peptide bonds. Some examples of peptides are: glutathione, galanin, oxytocin, bradykinin, amanitin, thyrotrophin, cholecystokinin, vasopressin and enkephalin.
amino acids
First of all, it is worth remembering that the amino acids are organic molecules formed by an amine group - NH2 and a carboxyl group - COOH, which are considered the basic units of peptides and proteins.
Thus, a set of amino acids form the proteins, used in their synthesis. They are classified into natural and essential amino acids, where the first is synthesized by the body itself and the others are those found in nature, that is, in food.
Proteins
At proteins they are macromolecules formed by the chain of amino acids. They are extremely important compounds for the proper functioning of the body, being basically formed of carbon, hydrogen, nitrogen and oxygen.
In such a way, peptides are considered small proteins, that is, they are fragments of proteins and, therefore, they are constituted by smaller numbers of amino acids in relation to proteins.
Also know about the Protein Structure.
Functions of Peptides
Like proteins, peptides are chemical compounds that designate several essential functions for life, namely:
- Regulates the activity of multiple systems
- Aids in DNA synthesis
- Amino Acid Transport
- Metabolism of drugs and toxic substances
- cell regeneration
- Anti-inflammatory effect
- Appetite stimulation or inhibition
- Stimulates urine production
- Regulates hormonal and neurotransmitter activity
- immune function
- natural antibiotics
Peptide Link
Peptide bonds are covalent chemical bonds (molecular bonds) that occur between two or more peptides, through the reaction between a carboxylic acid (-COOH) and an amine group (-NH2), releasing a water molecule (H2O) in a process called dehydration synthesis. Thus, a hydrogen (H) coming from the amine group unites with a hydroxyl (-OH) from the carboxylic group, forming the water molecule.
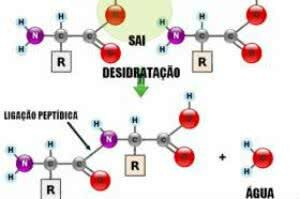 Peptide Link
Peptide Link
On the other hand, to break or undo a peptide bond, it is enough to add a molecule of water that will occur the opposite process to dehydration, called hydrolysis.
It is worth remembering that peptides are biomolecules formed by two or more amino acids and the union of many peptides make up proteins. In summary, the peptide bonds form proteins, essential for the proper functioning of the body.
Nomenclature
According to the number of amino acids present in the molecule, the peptides are classified into:
- Dipeptide: formed by two amino acids
- tripeptide: formed by three amino acids
- tetrapeptide: formed by four amino acids
- oligopeptide: from 4 to 50 amino acids
- Polypeptide: made up of more than 50 amino acids


