Volumetric analysis or volumetry it is a laboratory procedure in which we use a certain volume of a solution in concentration known to determine the concentration of another solution. The volume of the solution of known concentration will be determined when it completely reacts with the solution of unknown concentration, that is, the solutions involved must react with each other.
The instruments most used to measure a given volume are:
- Pipette

Example of pipette used in volume measurement
- Burette

The burette is an important volumetric measurement equipment
The volume measurement is made by evaluating the height of the so-called meniscus, which is nothing more than the surface region of the liquid, which presents a rectangular or domed aspect (drop shape), depending on the thickness of the container. The wider the container, the more rectangular the meniscus; the thinner the container, the more convex it will be. To assess the meniscus, the eye must be exactly at its height and we must use the lower region as a reference, if the meniscus is rectangular, or the tip, if bulged. See a representation of an assessment:
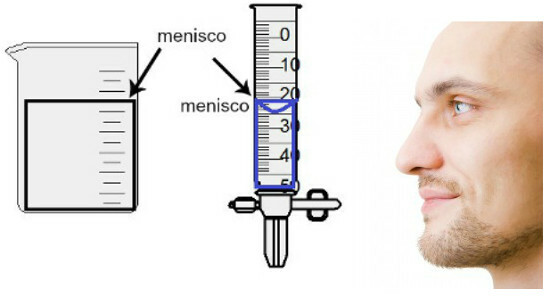
To assess a meniscus, it is important that your eyes are looking towards it.
In volumetric analysis, the most used equipment is the burette. This is because, as the method involves chemical reactions and these can be processed quickly, the burette allows the liquid is released at its tip in drops, which makes it possible for us to stop the liquid from coming out more controlled.
One of the most used procedures within volumetry is the titration. It is a volumetric analysis that involves the occurrence of a reaction between an acid and a base or vice versa. The equipment required for its realization is represented in the image below:
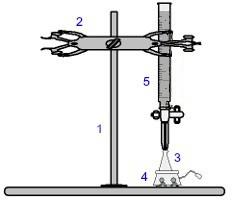
Representation of the equipment used in a titration
The numbers in blue mean:
1) Universal support;
2) Claw (used to hold the burette);
3) Erlenmeyer (receives the solution of unknown concentration);
4) Magnetic stirrer (used to stir the solution present in the Erlenmeyer flask);
5) Burette (receives the solution of known concentration).
A volume of the solution of unknown concentration with the phenolphthalein indicator is added to the erlenmeyer flask (immediately the solution will turn pink). In the burette, a certain volume of the solution of known concentration is placed. Then, the acid solution from the burette is directly dripped into the basic solution in the Erlenmeyer flask. This drip lasts until the base solution becomes colorless, at which point we call the turning point, which indicates that all the base present in the solution has completely reacted with the acid.
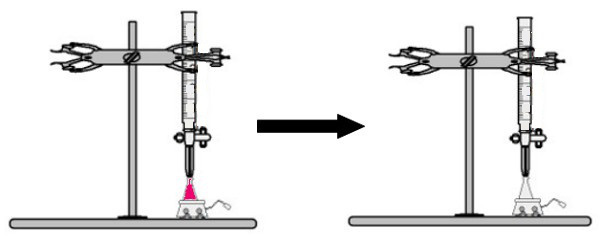
At the turning point, the solution is no longer pink and becomes colorless
Observation: If the solution of unknown concentration is acid, when it receives the phenolphthalein indicator, it will be colorless. Thus, the turning point in the titration will be when the acidic solution turns pink when receiving the basic solution from the burette.
Finally, to determine the concentration of the basic solution, just use the following equation:
Ma.Va = Mb.Vb
Ma = acid molarity;
Va = acid volume;
Mb = base molarity;
Vb = base volume.
Now follow an example of how the calculation of a solution with unknown molarity is performed.
1st) 30 mL of an unknown base solution was added to an Erlenmeyer flask in order to perform a titration to determine its concentration (molarity). Into the burette were added 50 ml of an acid solution with a concentration of 0.2 mol/L. After dropping the acid solution and until the basic solution was titrated, it was observed that 20 mL of the acid solution were used in the titration. Determine the molarity of the base solution used.
Exercise data:
Vb = 30 mL
Mb = ?
Ma = 0.2 mol/L
Va = 20 mL
As the molarity of the acid and its volume are, respectively, 0.2 mol/L and 20 mL, and the volume of the base used is 30 mL, just use the titration formula:
Ma.Va = Mb.Vb
0.2.20 = Mb.30
4 = Mb.30
4 = Mb
30
Mb = 0.133 mol/L
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/analise-volumetrica.htm
