An isobaric transformation occurs when the gas is at a constant pressure. For example, if done in an open environment, the transformation will be isobaric as the pressure will be atmospheric pressure that will not change.
In this case, temperature and volume vary. Two leading scientists have studied how this variation in isobaric transformations occurs. The first to relate the volume and temperature of gases was Jacques Charles (1746-1823), in 1787, and then, in the year 1802, Joseph Gay-Lussac (1778-1850) quantified this relationship.
Thus, a law emerged that explains the isobaric transformations of gases, which became known as the Charles/Gay-Lussac Law. It is stated as follows:
"In a constant pressure system, the volume of a fixed mass of a gas is directly proportional to the temperature."

This means that if we double the temperature, the volume occupied by the gas will also double. On the other hand, if we decrease the temperature, the gas volume will also decrease in the same proportion.
This can be seen in a very simple experiment. If we place a balloon in the neck of a bottle, a fixed mass of air will be trapped. If we dip this bottle into a bowl of ice water, the balloon will deflate. Now, if we put it in a bowl of hot water, the balloon will fill.
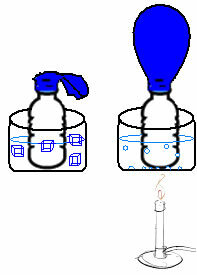
This is because as the temperature increases, the kinetic energy of the gas molecules increases and the speed at which they move also increases. Thus, the gas expands, increasing the volume it occupies, and the balloon inflates. The opposite occurs when we lower the temperature, putting it in cold water.
Do not stop now... There's more after the advertising ;)
This relationship between temperature and volume in isobaric transformations is given by the following relationship:
V = k
T
"k" is a constant, as can be seen in the following graph:
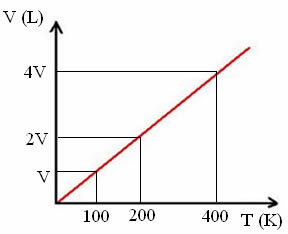
Note that the V/T ratio always gives a constant:
_V_ =_2V_ = _4V_
100 200 400
Thus, we can establish the following relationship for isobaric transformations:
Vinitial = VFinal
Tinitial TFinal
This means that when there is any change in the temperature of the gas at constant pressure, we can find out its volume through this mathematical expression. The opposite is also true, knowing the volume of the gas, we find out what temperature it is at. See an example:
"A gaseous mass occupies a volume of 800 cm3 at -23°C, at a given pressure. What is the temperature recorded when the gaseous mass, at the same pressure, occupies a volume of 1.6 L?”
Resolution:
Data:
Vinitial = 800 cm3
Tinitial = -23 ºC, adding to 273 we have 250 K (Kelvin)
VFinal = 1.6 L
TFinal = ?
* First we have to leave the volume on the same unit. It is known that 1 dm3 equals 1 liter. like a 1 dm3 is the same as 1000 cm3, it appears that 1 liter = 1 000 cm3:
1 L 1000 cm3
x 800 cm3
x = 0.8 L
* Now we replace the formula values and find the final temperature value:
Vinitial = VFinal
Tinitial TFinal
0,8_ = 1,6
250 TFinal
0.8 TFinal = 250. 1,6
TFinal = 400
0,8
TFinal = 500K
* Moving to the Celsius scale, we have:
T (K) = T (°C) + 273
500 = T (°C) + 273
T (°C) = 500 - 273
T (°C) = 227°C
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Isobaric Transformation"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/transformacao-isobarica.htm. Accessed on June 27, 2021.
What are gases, what are the properties of gases, molecular compounds, compressibility, fixed volume, kinetic energy mean, absolute temperature of a gas, ideal gas, Real gases, perfect gas, gas state variables, volume of a gas, seasons
Chemistry

Gay-Lussac Laws, Proust Law, Chemical Reaction, Constant Proportion, Masses of Substances, Pure Substance, Analysis qualitative and quantitative, law of perfect gases, law of constant proportions, law of definite proportions, law volumetric.