Thus, if we connect 6 batteries of 2.0 V each, we will get a battery with a capacity of 12 V.
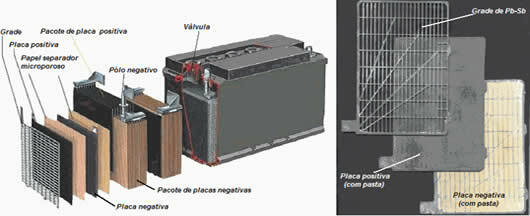
Car batteries normally have this electromotive force of 12 V, as they are composed of 6 lead-acid cells or cells. And they are also called as lead batteries, because its anode (negative pole) is lead plates and its cathode (positive pole) is lead plates with oxide and lead IV (PbO2).
These batteries have high currents, which allow starting motors thanks to their high power density values.
As seen in the figure below, the PbO-coated lead plates2 (negative plates) are connected to the positive connector. While lead plates (positive plates) are connected to the negative connector. They are separated by some cardboard, plastic or some microporous separating paper.
This set is placed in the battery compartment and immersed in an aqueous solution of sulfuric acid (H2ONLY4) with a density of approximately 1.28 g/cm3.
The semi-reactions and the global reaction that take place in this battery are:
Anode: Pb +HSO
41-+ H2O ↔ PbSO4 + H3O1+ + 2e-Cathode: PbO2 + HSO41-+ 3H3O1+ + 2e-↔ PbSO4 + 5 hours2O________
Overall reaction: Pb + PbO2 + 2 HSO41-+ 2 H3O1+↔ 2 PbSO4 + 4 H2O
As can be seen from the double arrow above, these reactions are reversible, which means it is possible recharge lead batteries again by supplying energy to the system, that is, it is possible to pass an electrical current supplied by a direct current generator. In this way, the direction of these reactions is reversed, with the regeneration of a large part of the sulfuric acid taking place and thus charging the battery. In the car, this potential difference that supplies energy and recharges the battery is made by the dynamo or by alternator.
Sulfuric acid density helps identify if the battery is depleted. Since its density is 1.28g/cm3; if this value is below 1.20 g/cm3, means that sulfuric acid has been consumed and the battery is discharged. Hence, these batteries are very durable.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
| Batteries are a set of cells connected to each other, in series, that is, the positive pole of one cell is connected to the negative pole of another, and so on. |
Source: Brazil School - https://brasilescola.uol.com.br/quimica/bateria-chumbo-dos-automoveis.htm
