As explained in the text Physical States of Water and its Changes, the change from solid to liquid is called Fusion. Consequently,the melting point is the temperature at which a pure substance that was in a solid state changes to a liquid state.
If you take an ice cube that was in the fridge at a temperature of -5°C and leave it outside on a hot day, what will happen? It's simple, the ice cube will start to melt. But that won't happen until after you've taken it out of the fridge. This is because the ice temperature starts to increase, going from -5 ºC to -4 ºC, then to -3 ºC and so on until it reaches 0 ºC. This is the temperature at which ice starts to melt. So we can say that the melting point or melting temperature of ice is equal to 0°C (at sea level).
*Remembering that ºC means degrees Celsius, which is a measure of temperature.

melting ice or melting
The most interesting thing is that the temperature remains at the same value throughout the physical state change. This means that, in the case of ice, after it reaches the melting point, its temperature stays fixed at 0 °C until all the ice changes to a liquid state.
This can be visualized in a graph like the one shown below. Note that, until reaching the melting temperature, the temperature continued to rise, but when this temperature was reached, it remained fixed over time, forming a plateau:

Ice melting point graph
The boiling point is the temperature at which a pure substance changes from a liquid to a vapor state. This is what happens when we put the liquid water that comes out of the tap into the fire. Its temperature increases until it reaches a temperature of 100ºC, which is the boiling point of water (at sea level).
In the following figure, you can see that when the liquid is heated, an increase in agitation occurs. of its particles, which allows them to escape out of the container and mix with the air. The bubbles formed at the boiling temperature are water vapor bubbles.
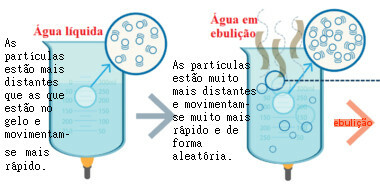
Movement of boiling water particles
At the boiling point, the same happens as at the melting point, that is, the temperature remains at the same value during the change of state.
The following graph shows the melting point (MP) and boiling point (PE) of water:

Physical state change graph of water at sea level
Melting and boiling points are important properties of substances, as each has a specific melting temperature and boiling temperature. There are no two or more substances with the same melting and boiling points. The only known substance with a melting point of 0°C and a boiling point of 100°C at sea level is water.
Therefore, these quantities are used to identify substances. See some melting and boiling point values for other substances:

Melting and boiling points of some materials present in everyday life
However, it is noteworthy that the melting and boiling temperatures will only have fixed values if the substance is pure. In the case of mixtures, these values vary, that is, they boil at one temperature and end up changing their physical state at another. Thus, the graphs of changes in the physical state of mixtures do not have those two fixed thresholds for the melting point and for the boiling point.
By Jennifer Fogaça
Graduated in Chemistry