THE áwater, precious element for humanity, presents three physical states when it comes into contact with different temperatures. These states are:
• liquid;
• solid;
• gaseous.
In each of the physical states of water, the constituent particles of matter are aggregated in different ways.
Read too: How important is water to our body?
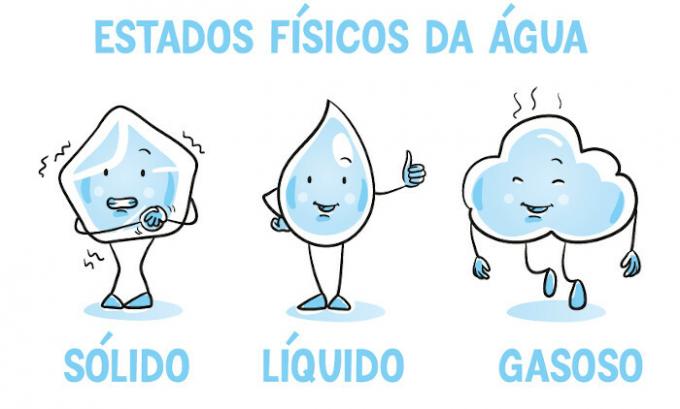
What are the physical states of water?
Liquid
In the liquid state, water takes the shape of the container in which it is stored, whether in nature, as is the case of lakes, whether in the daily lives of human beings, as in the bottles we buy in the supermarket.

Gaseous
In the gaseous state, water has no shape. At this stage she goes through the evaporation process, when it leaves the liquid state to enter the gaseous state. This happens when water is heated to a boil and starts to evaporate. When water is naturally heated by heat, it evaporates in the atmosphere and forms droplets, which give rise to cloudandus.

Solid
In the solid state, water has regular way. That happens
when it comes into contact a lower temperature and so it solidifies. We find this state of water in ice, iceberg, snow and hail.
See too: Why is sea water salty?
How are the water state changes?
As we have already seen, water undergoes changes when placed in different situations. But do you know how each change happens? Let's learn now!
Solidification
And the transition from liquid to solid state. This is only possible when the water reaches a temperature of 0°C. This happens when we put water in the freezer and it turns to ice.
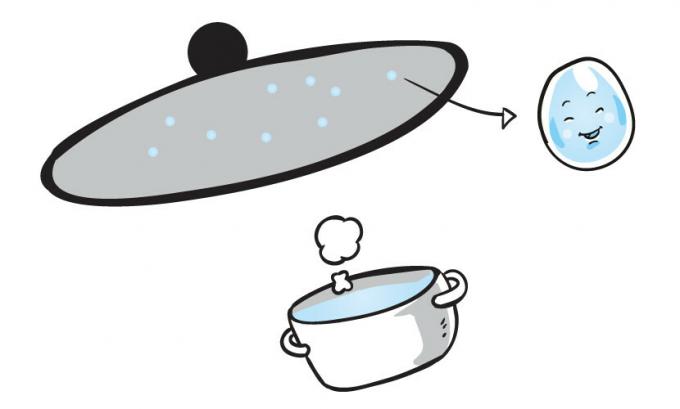
Condensation
And the change from gas to liquid state. We can notice this change when cooking food. The water in the pot boils and forms small drops of water on the lid of the pot.
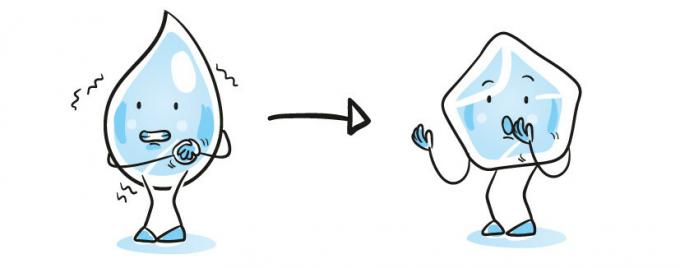
Fusion
And the change from solid to liquid state. This happens, for example, when we take the ice out of the freezer and put it at room temperature. Remember that for water to turn to ice, it needs to reach a temperature of 0°C. At temperatures greater than 0°C, ice begins to melt.
Sublimation
Finally, we have sublimation, which happens when water goes straight from a gaseous state to a solid state, without having to go through the liquid state. This phenomenon rarely happens in nature, as water generally follows a natural order, from liquid to solid or from liquid to gas.

See too: Why does the skin on the feet and hands wrinkle in water?
Activity
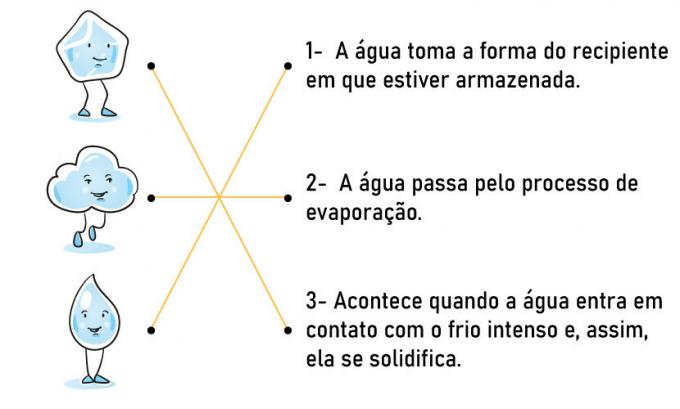
1) Match the images with their proper descriptions.

Reply: