O fire it has been part of human history for many, many years. With it, it was possible to better prepare food, manufacture materials in general, in addition to obtaining heating in cold periods. Has it changed over the centuries? Certainly not.
O fire is related to the most diverse activities in our daily lives. How many materials for our daily use were produced with his help? There is no denying it! Without him, our days would be much more difficult. But... have you ever stopped to think about your physical condition?
To answer this question, it is first necessary to understand some extremely important concepts within the study of Chemical Science. These concepts are matter, the physical states of matter and energy.
→ Matter, physical states of matter and energy
Matter it is everything that occupies space and presents mass. When we put water in a bottle, for example, it starts to occupy the space of the bottle and, by this, we easily notice that the mass of the bottle is increased, that is, water is an example of matter. See other story examples:
Air
Earth
trees
Liquids
solids
Gases
Glass
Metals
The states in which we can find matter basically are:
Solid (the particles that make up matter are very close);
Liquid (when the particles that make up matter are less close together);
Gaseous (when the particles that form matter have no aggregation);
Plasma (when the atoms that form matter are free, at high temperatures).
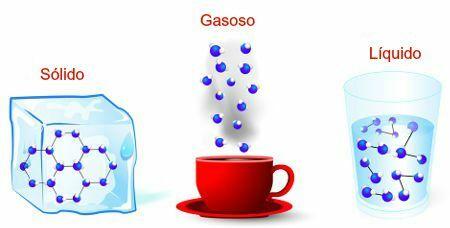
Representation of solid, liquid and gaseous states
THE energy, in turn, is defined as a force capable of performing work, action or movement, neither occupying space nor presenting mass. A classic example of energy is light. We cannot determine its mass on a scale and we know that it does not take up space in the environments we are in. Other examples of energy are:
Electricity
Sound
Heat
Cold
→ What, then, is the PHYSICAL STATE OF FIRE?
In fact, fire has no physical state, as it is not matter. Fire does not take up space and has no mass, so it cannot be solid, liquid, gas or plasma. Therefore, only the energy option was left. That's right, fire is energy. In fact, fire is a mixture of two energies:
Light
Heat
Proving this is very easy, just look at a lighted candle. When we light the candle in a dark environment, the light illuminates the place and, if we get closer, we will feel the heat that emanates from it. And one thing is for sure: it will not be possible to remove neither light nor heat from the environment, since they are forms of energy.

Lighting a candle proves that fire is a mixture of energies
ATTENTION: One thing also has to be very clear: To produce fire, some materials are needed (oxygen and fuel), which shows us that all matter has energy, but energy is not matter.
By Me. Diogo Lopes Dias