Scientific notation is a form of write numbers in a simplified way. It can be used to abbreviate both very large numbers and very small numbers.
The key to solving scientific notation is translating the number to a power of base 10 (10x).
How to make a scientific notation?
See the formula for scientific notation:
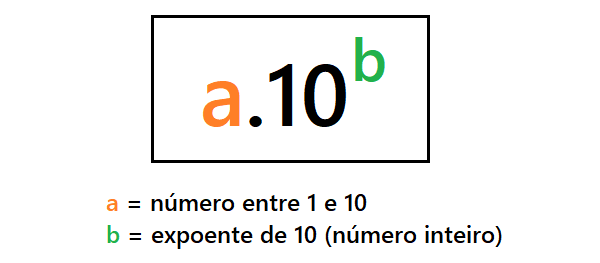
To transform a number into scientific notation, follow these steps:
- Write the number in decimal form. Only a digit other than 0 must be before the comma, that is, it must be a real number between 1 and 10 (example: 1.5).
- Count how many decimal places the comma went.
- Put this number of places as an exponent of 10. It is necessary to be careful when walking with the comma: if the number decreases, the exponent will be positive (example: 102). If the number increases, the exponent will be negative (example: 10-3).
To better understand, see the example with the number 180000:
- Bring the comma between the numbers 1 and 8, to get a number between 1 and 10.
- Count how many decimal places the comma was moved to reach this position. In this example there were 7 houses.
- Put the number 7 as a power of 10.
This is the result of the number 18000 written as scientific notation: 18000000 = 1,8. 107.
Other examples of scientific notation
1900 = 1,9.103
33000 = 3,3.104
28900000 = 2,89.107
0,0000000022 = 2,2.10 - 9
What is scientific notation for?
Scientific notation can be used to facilitate calculations involving very large or very small numbers. It can be applied in many areas, but it is more common in sciences such as mathematics, physics and chemistry.
See these examples:
- 150000000 km is the distance between the Earth and the Sun (1.5.108)
- 1427000000 km is the distance from Saturn to the Sun (1,427.109).
- 0.000000000000000000000000167252 g is the mass of a proton (1.67252.10-24).
- 0.00000000000000000000000000091091 g is the mass of an electron (9.1091.10-28).
Mathematical operations (addition, subtraction, multiplication and division) also use scientific notation. See the demonstration of some exercises:
Addition
To solve a scientific notation in addition operations it is necessary to add the coefficients. The exponents must also be added together and the result is the exponent of 10. See how to do it:
3,1.103 + 6.103 = (3,1 + 6).103 = 9,1. 103
Subtraction
To solve a scientific notation into a subtraction, the process is similar to adding. In that case, the coefficients must be subtracted. The exponents also need to be subtracted and the result is the exponent of 10. Look at the example:
8 .10-4 – 2 .10-4 = (8 – 2).10-4 = 6 .10-4
Multiplication
In multiplication that contains scientific notations, the coefficients must be multiplied. Already the exponents must be added and the result must be placed as an exponent of the number 10. Look:
(4 .103).(2 .106) = (4.2).103+6 = 8.109
Division
In divisions with scientific notation, the coefficients must be divided. The exponents must be subtracted and the result is the exponent of the number 10. Watch:
12.106 ÷ 4 .103 = (12÷4) .106–3 = 3 .103
Also know the meaning of Pi (π), Prime number and MMC and MDC.

