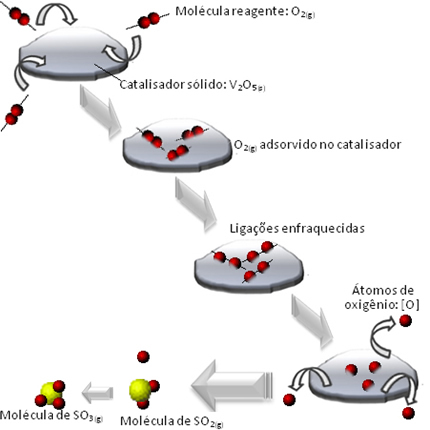
O shifting a chemical equilibrium is the way in which a reaction system gets out of a situation of chemical balance. In this shift, the speed at which the forward reaction (arrow 1) takes place is the same as the reverse reaction (arrow 2).
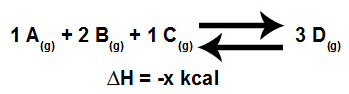
Model of an equation in chemical equilibrium
So, during the shifting a chemical equilibrium, the inverse reaction prevails over the direct reaction and tends to form reactants A, B, C or the direct reaction prevails over the inverse reaction and tends to form the product D.
These occurrences were reported by the French chemist Henri Louis Le Chatelier. He found that when a system in equilibrium is disturbed, it tends to work against the disturbance generated and, for that, seeks to reach a new equilibrium situation. This system trend is called principle of Le Chatelier.
According to studies carried out by Le Chatelier, the only factors capable of promoting shifting a chemical equilibrium they are:
Participant concentration;
Temperature;
Pressure.
Influence of concentration on balance shift
The change in concentration of a reaction participant is a factor that can promote the shifting a chemical equilibrium. In general, according to Le Chatelier's principle, with regard to changing the concentration of one of the participants, the balance behaves as follows:
Increased concentration: the balance moves in the opposite direction to the participant;
Decrease in concentration: the balance moves in the same direction as the participant.
Equilibrium relationship example:
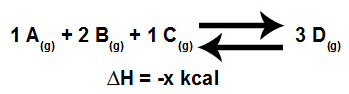
Model of an equation in chemical equilibrium
So, if:
We increase the concentration of reagents A, B or C: the balance will be shifted in the opposite direction to them, that is, it will be shifted to the right (direction of formation of D);
We increase the concentration of product D: the balance will be shifted in the opposite direction to that of the reactants, that is, it will be shifted to the left (direction of the formation of reactants A, B and C);
We decrease the concentration of reagents A, B or C: the equilibrium will be shifted in their same direction, that is, it will be shifted to the left (direction of the formation of reactants);
We decrease the concentration of product D: the balance will be shifted in the same direction as it, that is, it will be shifted to the right (direction of the formation of the product).
Note: Changing the concentration of solid participants does not shift the balance.
Influence of temperature on equilibrium displacement
Changing the temperature during a chemical reaction is a factor that can promote the shifting a chemical equilibrium. In relation to this change in temperature, in general, according to Le Chatelier's principle, the balance behaves as follows:
Do not stop now... There's more after the advertising ;)
In rising temperature: balance shifts towards endothermic reaction;
In decreasing the temperature: the balance shifts towards the exothermic reaction.
To perform the analysis of the influence of temperature on an equilibrium, it is essential to know the nature of the direct and inverse reactions, which is determined by the variation in the reaction enthalpy. So, if:
∆H positive: endothermic direct reaction and exothermic inverse reaction;
∆H negative: exothermic direct reaction and endothermic inverse reaction.
For example, regarding the following balance:
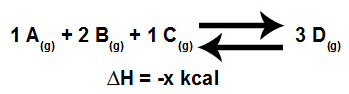
Model of an equation in chemical equilibrium
In this case, by having negative ∆H, the forward reaction is exothermic and the reverse reaction is endothermic. So, if:
We increase the system temperature, the equilibrium will be shifted in the opposite direction (of the endothermic reaction), that is, it will be shifted to the left (the direction of the formation of reactants);
We lower the system temperature, the equilibrium will be shifted in the direct direction (of the exothermic reaction), that is, it will be shifted to the right (the direction of the formation of product D).
Influence of pressure on balance displacement
During a chemical reaction, the change in pressure in the environment is a factor that can promote the shifting a chemical equilibrium. In general, according to Le Chatelier's principle, balance behaves as follows with (a):
pressure increase: the balance moves towards the smallest volume;
Decrease in pressure: balance shifts towards greater volume.
To analyze the influence of pressure on an equilibrium, it is essential to know the volume established in the reactants and products, which can be determined by the coefficients that make the equation balanced, as in the example follow:
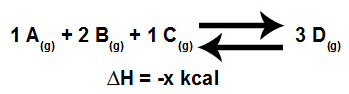
Model of an equation in chemical equilibrium
Thus, the reagents have a volume of 4L, which is obtained by the sum of coefficients 1, 2 and 2, and the product, which is unique, has a volume of 3L (given by coefficient 3).
Thus, in relation to the balance above, if:
We increase system pressure, the equilibrium will be shifted in the direct direction (from the smallest volume, 3L), that is, it will be shifted to the right (direction of the formation of product D).
We decrease system pressure, the equilibrium will be shifted in the opposite direction (from the largest volume, 4 L), that is, it will be shifted to the left (the direction of the formation of reagents).
Note: Increasing or decreasing pressure in an equilibrium system can promote displacement only in situations where the volume of reactants is different from the volume of products.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Displacement of chemical balance"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/fatores-que-alteram-equilibrio-quimico.htm. Accessed on June 28, 2021.
Chemistry

Test your knowledge and learn more with this list of solved exercises on chemical balances. Through this material, you will be able to better understand how to work equilibrium constants (Kp, Kc and Ki), equilibrium shift, pH and pOH, as well as equilibrium in so-called buffer solutions.