In the text Isomers in molecules with different asymmetric carbons two formulas proposed by Van’t Hoff and Le Bel were shown that help to determine the amount of optical isomers when the molecule has two or more different asymmetric carbons.
But when the molecule has several equal asymmetric carbons it is not possible to use these formulas. We determine if a molecule is like this if it has two or more carbons that have their ligands different from each other, but exactly the same as the ligands of another carbon(s).
For example, tartaric acid is a substance that is formed during the process of fermenting grape juice for the purpose of producing wine. Your molecule has two equal asymmetric carbons, both of which have the following ligands:
│
HOOC─, H─HO─ and H─C─OH
│
COOH
In the projections below, we have two possibilities: in the first, the OH ligands are on opposite sides (and the H ligands too); in the second, these ligands are on the same side and in each of the possibilities we have the respective mirror images:

Since the two asymmetric carbons are equal, the angle α of deviation from the plane of polarized light will be the same. So, in the first case we have that, because the conformation of the carbon atoms is different, one will shift the light plane polarized to the right (right-hand) at angle +α, and the other will shift the plane of polarized light to the left (levorotator) at the angle –α. This means that one will nullify the effect of the other and the substance will be optically inactive by internal compensation, being called compound meso.
Do not stop now... There's more after the advertising ;)
In the second possibility shown above, both carbons have the same conformation, so two cases of optically active compounds:
1st) the two asymmetric carbons shift the polarized light plane to the right (dextrorotatory): +2α;
2nd) the two asymmetric carbons shift the polarized light plane to the left (levorotator): -2α.
Tartaric acid can also form a racemic mixture that has 50% of the dextrorotatory isomer and 50% of the levorotary isomer. One isomer will nullify the deviation of the polarized light plane of the other, therefore, this mixture is considered optically inactive by external compensation.
With that, we come to the conclusion that a molecule that has two equal asymmetric carbon atoms has two optically isomers. active (the dextrogyrus and the levogyrus) and two optically inactive isomers (the meso compound and the racemic mixture), as shown in the summary bellow:
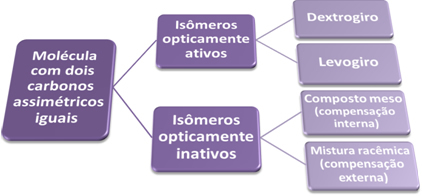
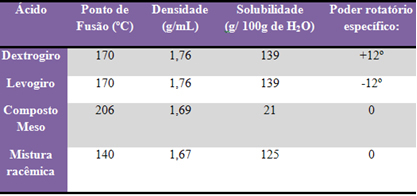
These different isomers have the same molecular formula, however, their properties differ, see:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Molecule with equal asymmetric carbons"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/molecula-com-carbonos-assimetricos-iguais.htm. Accessed on June 28, 2021.