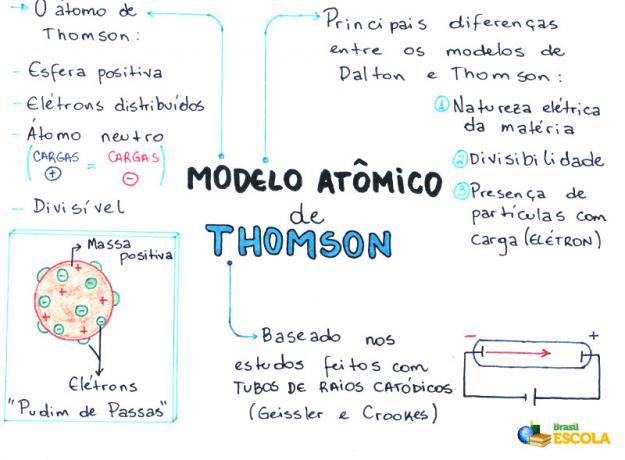
O Thomson atomic model was proposed in the year 1898 by the English physicist Joseph John Thomson or, simply, J.J. Thomson. After having several experimental evidence for the existence of the electron, he overturned the theory of the indivisibility of the atom proposed by John Dalton.
Thomson, based on his model, confirmed and proved the existence of electrons (particles with a negative electrical charge) in the atom, that is, the atom has subatomic particles.
Mind Map: Thomson Atomic Model
* To download the mind map in PDF, Click here!
Thomson proposed his atomic model based on discoveries related to radioactivity and experiments carried out with the cathode ray tube built by scientists Geissler and Crookes. See a representation of this tube:
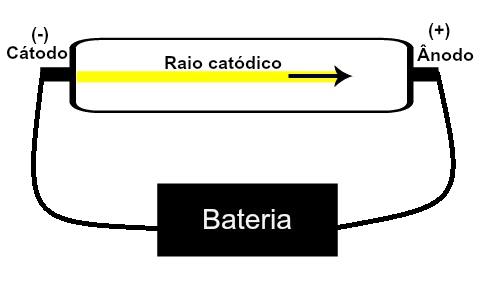
When a rarefied, low-pressure gas is subjected to a high electrical voltage (eg 15000 V), it produces a beam of light (composed of electrical charges) from the cathode (negative pole) towards the anode (pole positive).
With this experiment, Thomson came to the conclusion that when the atoms of the gaseous material inside of the tube were subjected to a high voltage, their electrons were ripped out and directed to the plate positive.
Considerations proposed by the Thomson atomic model
With the experiments carried out with the cathode ray tube, Thomson proposed his interpretation of what the atom and its constitution would be like. So, according to him:
- The atom is a sphere, but not massive as proposed by the John Dalton atomic model;
- The atom is neutral, since all matter is neutral;
- As the atom has electrons, which have negative charges, therefore, it must have positive particles so that the final charge is null;
- Electrons are not fixed or trapped in the atom, they can be transferred to another atom under certain conditions;
- The atom can be considered as a continuous fluid with positive charges where electrons, which have a negative charge, would be distributed;
- associated your model to a raisin pudding (which represent the electrons);
- Since the electrons that are scattered have the same charge, there is a mutual repulsion between them, which makes them evenly distributed in the sphere.
Novelties proposed to the atom by the Thomson model
Thomson's atomic model was the second proposed for the atom. The first model was formulated by John Dalton.
Do not stop now... There's more after the advertising ;)
Thomson's model dealt with new knowledge about the atom that until then had not been proposed for lack of scientific basis, such as:
- Electrical nature of matter;
- Atom splitability;
- Presence of small, charged particles in the atom.
Problems pointed out to the Thomson atom
Several physicists at the time of the proposal of the Thomson atomic model, based on the theories of Classical Physics, pointed out some inconsistencies present in this model:
- Thomson proposed that the atom had a stability in relation to the uniform distribution of electrons, which could be modified by the influence of energy. However, Classical Physics, based on the electromagnetism, does not allow the existence of a stable system based only on the repulsion between particles of the same charge;
- For Thomson, electrons are evenly distributed in the atom, but they have the ability to shift in an accelerated way and, therefore, must emit electromagnetic radiation at certain frequencies specific. However, this was not observed.
- Thomson's model was often ineffective in explaining atomic properties, such as their composition and organization.
* Mind Map by Victor Ricardo Ferreira
Chemistry teacher
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Thomson's Atomic Model"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/o-atomo-thomson.htm. Accessed on June 27, 2021.
Chemistry

Niels Bohr, Bohr's atom, atomic physics, stable atom, atomic model, planetary system, layers of the electrosphere, energy levels, electron shells, electron energy, Rutherford atomic model, excited state atom.
Chemistry
Atoms and the construction of the Universe, atomic theory, that everything is made, matter is made up of atoms, Theory of the four elements, ancient alchemists, atomic theory, fundamental particle.