At stereoisomerism, or space isomerism, there is the optical isomerism, which occurs when compounds are optically active, that is, they bend the plane of polarized light.
Isomers that exhibit optical activity are called enantiomers, they have three main characteristics:
- Your molecule is asymmetric. This means that if we divide it in half, the resulting parts will not be equal;
- The enantiomers are the mirror image of each other;
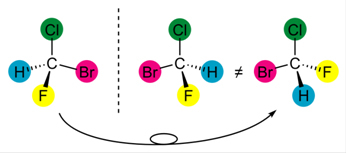
- They are not superimposable, that is, if we place one molecule above the other, they will not be equal, the arrangement of their binding atoms will be different.
These enantiomers, whose molecules are asymmetric and non-overlapping, are called chiral molecules, because the word "chiral" comes from the Greek coffer which means “hand”, because the hands are asymmetrical and not overlapping.

Also, if we place the left hand in front of the mirror, its image will look exactly like the right hand and vice versa. In other words, the hands are the same as the enantiomers, in the sense of being the mirror image of each other.
Do not stop now... There's more after the advertising ;)

Enantiomers differ from diastereoisomers due to the fact that the latter are not mirror images of each other. Both enantiomers and diastereoisomers are stereoisomers or space isomers.
However, the enantiomers are considered the most important, especially with regard to biochemical phenomena. The activity that each enantiomer exerts in the organism is different from one another.
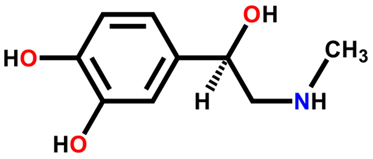
For example, the hormone adrenaline has two enantiomers, dextrogyro and levogyro. Dextrogyrus is often less active as a hormone than levogyrus. The action of levorotatory adrenaline is important because it acts as a powerful vasoconstrictor and hypertensive vessel, which has a pronounced effect on metabolism, causes increased heart rate and tension arterial.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "What are Enantiomers in Isomery?"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/o-que-sao-enantiomeros-na-isomeria.htm. Accessed on June 28, 2021.