They call themselves inner transition elements all 28 chemical elements located in the 6th and 7th period of group 3 (or family IIIB) of the Periodic table. They are positioned more specifically outside the main body of the Table.
are called transition elements because they do not belong to families A (representative elements), but they cannot be confused with the elements of external transition (elements belonging to families B and positioned in the main body of the Table Periodic).
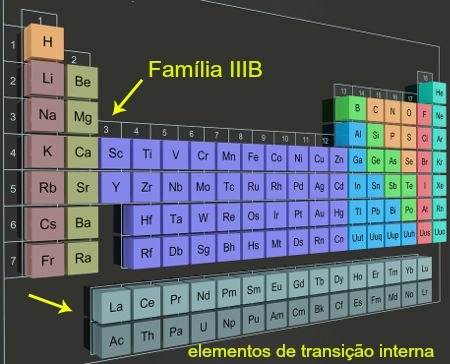
Internal transition elements in columns located outside the Periodic Table
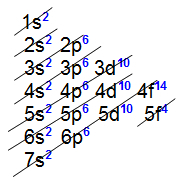
You inner transition elements present the f sublevel as more energetic. A proof of this is that each of the horizontal columns referring to these elements has only 14 elements, with 14 being the maximum number of electrons supported by sublevel f.
a) Lanthanide series
The lanthanides are all those that belong exclusively to the 6th period of the IIIB family, being so named because the first element in the series is lanthanum (La). See the names and acronyms of all of them:
Lanthanum (La)
Cerium (C)
Praseodymium (Pr)
Neodymium (Nd)
Promethium (Pm)
Samarium (Sm)
Europium (I)
Gadolinium (Gd)
Terbium (Tb)
Dysprosium (Dy)
Holmium (Ho)
Erbium (Er)
Thulium (Tm)
Ytterbium (Yb)
All internal transition elements present the 4f sublevel as the most energetic, that is, the eletronic distribution of all of them ends at this sublevel, as we can see in the distribution of two elements belonging to the series:
Do not stop now... There's more after the advertising ;)
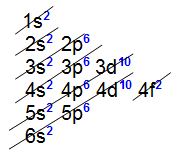
Electronic distribution of the lanthanide element Cerium

Electronic distribution of the lanthanide element Holmium
b) Actinide series
Actinides are all those that belong exclusively to the 7th period of the IIIB family, being so named because the first element in the series is Actinium (Ac). See the names and acronyms of all of them:
Actinium (Ac)
Thorium (Th)
Protactinium (Pa)
Uranium (U)
Neptunium (Np)
Plutonium (Pu)
Americium (Am)
Curium (Cm)
Berkelium (Bk)
California (Cf)
Einsteinium(es)
Fermium (Fm)
Mendelevium (Md)
Nobel (Nb)
All internal transition elements present the 5f sublevel as the most energetic, that is, the distribution electronics of all of them ends at this sublevel, as we can see in the distribution of two elements belonging to the series:
Electronic distribution of the Uranium actinide element

Electronic distribution of the California actinide element
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Internal Transition Elements"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/elementos-transicao-interna.htm. Accessed on June 27, 2021.