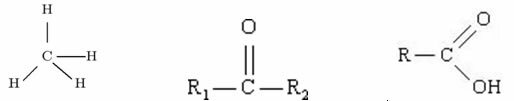Molecule is a set of atoms, the same or different, joined by covalent bonds.
These chemical species are electrically neutral and represent the forming unit of a substance.
There are simple molecules such as oxygen (O2) of the air we breathe. However, there are also complex compounds, such as buckyballs (60 carbon atoms bonded in a sphere shape), which are the largest molecules ever found in space.
Molecule Study
Covalent bonding in a molecule corresponds to sharing electrons, usually between non-metallic elements.
See the water molecule as an example of a simple compound.
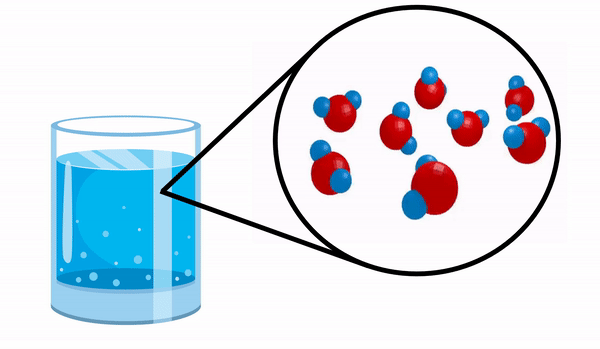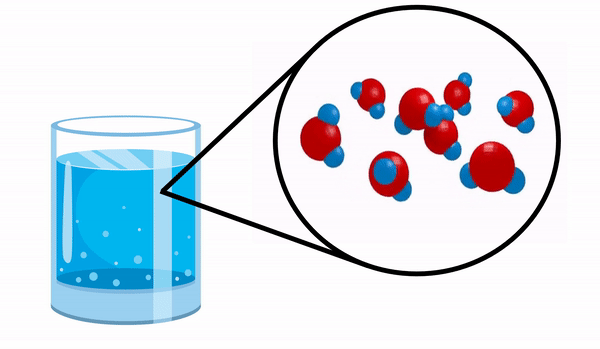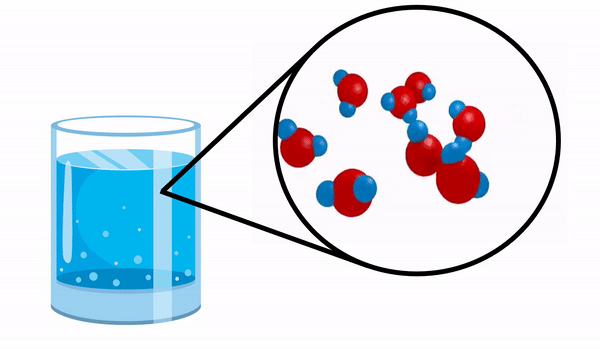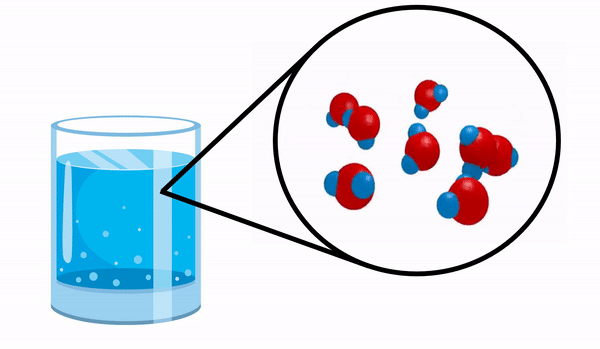
When we observe a glass with Water we have no idea that this substance is made up of several H molecules2O. This formula indicates that water is made up of 3 atoms: two hydrogen and one oxygen atoms, which are sharing electrons with each other.
Sugar, which we use to sweeten juices and make cakes, is also made up of molecules. The forming unit of sugar is sucrose.

This molecule is much more complex, as there are 45
atoms connected. It is formed by: 12 carbon atoms, 22 hydrogen atoms and 11 oxygen atoms.Molecules are structures of known molecular mass, but there are also macromolecules, which are "giant structures" formed by so many atoms that their composition is even undefined. An example of this type is diamond, a macromolecule formed by numerous atoms of carbon in a covalent network.
Covalent bond
A covalent chemical bond is established between two atoms when they share their electrons more external (of valence). Molecules can have two types of bonds:
Molecular covalent bond: the pair of electrons of the two bonding atoms are shared.

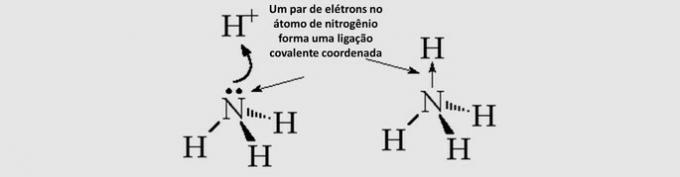
Covalent Covalent Bond (dative): the shared electrons come from only one of the atoms involved.
Molecular Geometry
When a molecule is formed, the atoms are positioned in different ways, so that the spatial arrangement is more stable. Therefore, composites have different geometries.
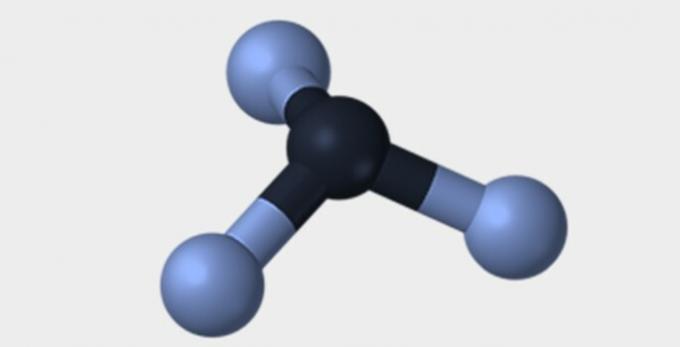
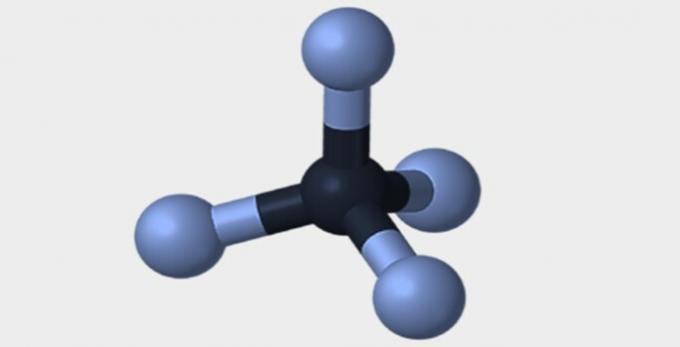
Here are some of the geometries that molecules can have.
| Molecular Geometry | ||
|---|---|---|
| Linear | Angular | Triangular |
 |
 |
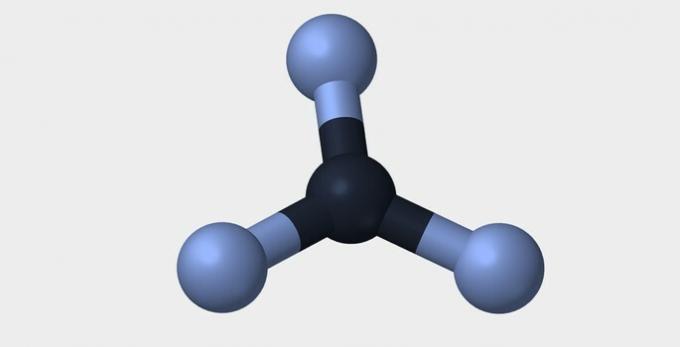 |
| Pyramidal | tetrahedral | octahedral |
 |
 |
 |
Polar and non-polar molecules
Molecules are classified according to the polarity.
nonpolar molecules: there is no difference in electronegativity between atoms.
| Nitrogen (N2) | carbon dioxide (CO2) |
|---|---|
 |
 |
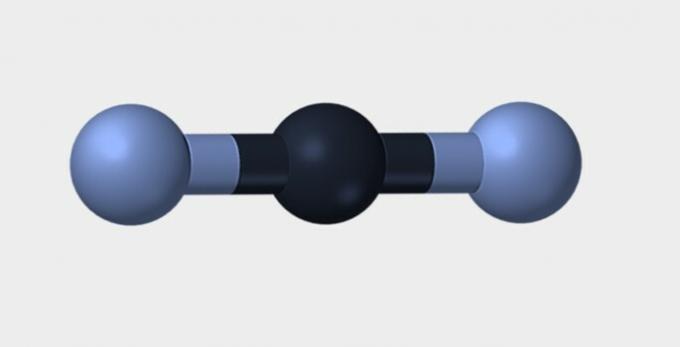
Nitrogen (N2) is a non-polar molecule because it is formed by it. chemical element and therefore there is no difference in electronegativity. carbon dioxide (CO2) is non-polar due to its linear geometry, which stabilizes the attraction of oxygen by electrons.
polar molecules: there is a difference in electronegativity between the atoms, with a positive pole and a negative pole.
| Water (H2O) | Ammonia (NH3) |
|---|---|
 |
 |
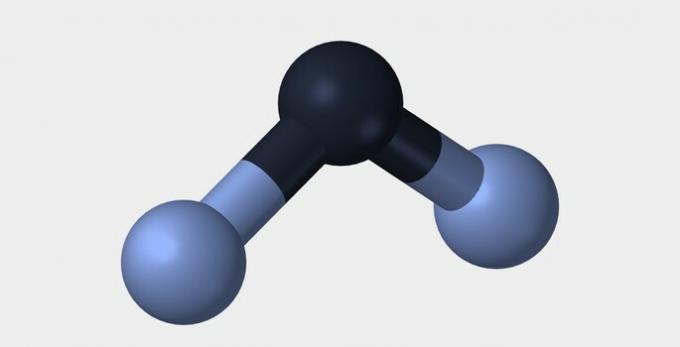
In both examples, we see that the central atoms, oxygen and nitrogen, have unpaired electron pairs that form electron clouds. Since there are more electronic clouds around the central atoms than there are established chemical bonds, the molecules are polar.
Examples of molecules
| Substance | Features | Molecule | Formula |
|---|---|---|---|
| Hydrogen | Fuel and abundant in the earth's crust. | 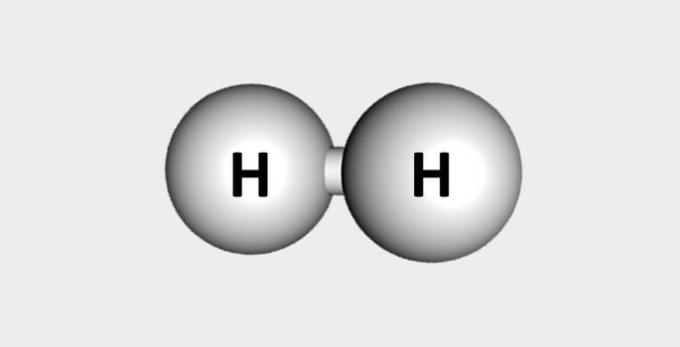 |
H2 |
| Oxygen | Essential for breathing and participates in various chemical reactions | 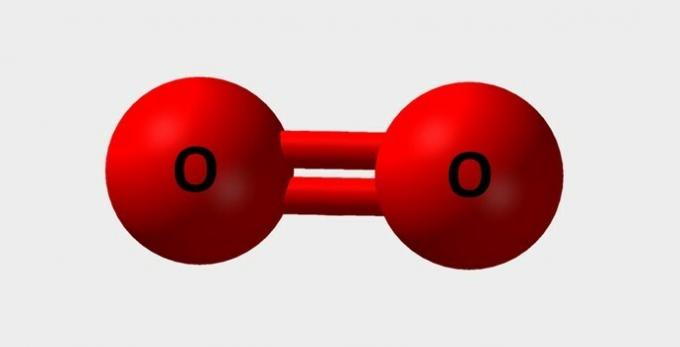 |
O2 |
| Sulfur | Yellow powder used to make dyes. | 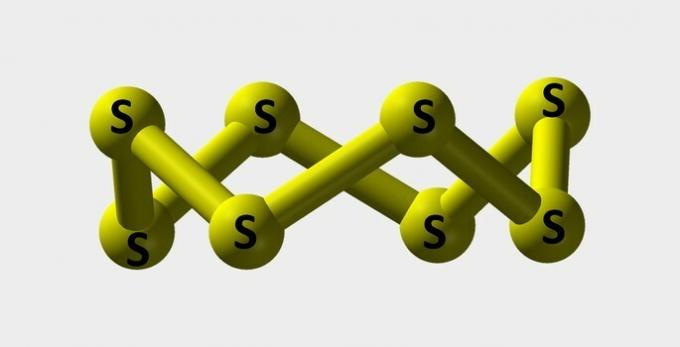 |
s8 |
| Carbon dioxide | Used in fire extinguishers and refrigerants. | 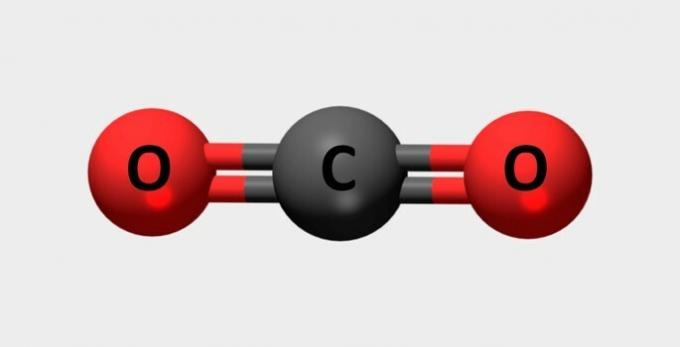 |
CO2 |
| Ethanol | Common alcohol used as fuel and in perfumes. | 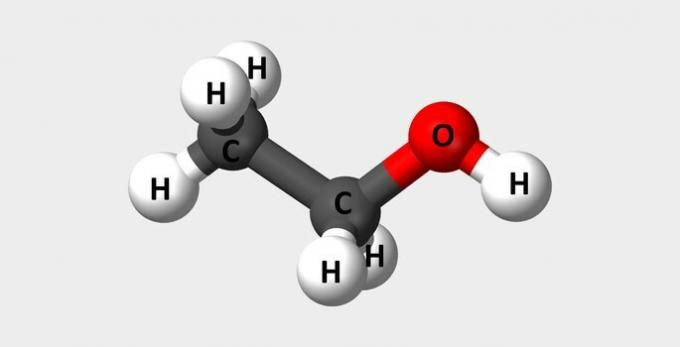 |
Ç2H6O |
Be sure to check out these texts on topics related to what you've just learned:
- Biomolecules
- Organic compounds
- Molecular mass
- Octet Rule
- Chemical bonds
- Connection Polarity
- Intermolecular Forces