THE electronegativity it is the tendency of an atom to attract electrons towards itself when it is linked to another chemical element. through a covalent bond, that is, in which electrons are shared, considering this molecule as being isolated.
Let's consider two examples to better understand the concept presented:
1st Example: Hydrogen gas molecule: H2 → H - H
When two hydrogen atoms come together, forces of attraction between the nucleus of each occur at the same time. one of these atoms by the electron of the other atom and repulsion forces between the electrons and the nuclei of the two atoms. When these forces reach equilibrium, the two electrons are in a region of the electrospheres that is somewhere between the two. atoms of the molecule, in which both interact with the two electrons, becoming stable, that is, the two atoms share a pair of electrons.
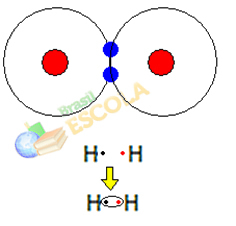
This is a covalent bond, which forms a molecule. But since the two atoms of this molecule are exactly the same, the way they attract electrons to each other is also the same. So we say that
there is no electronegativity difference or that she it's apolar.2nd Example: Hydrogen chloride molecule: HCℓ
In this case, the sharing of an electron pair is carried out between different elements, because, in this connection, the chlorine atom attracts electrons with greater intensity than hydrogen. Therefore, we say that chlorine is more electronegative than hydrogen.
As shown in the figure below, due to the difference in electronegativity, a electric dipole (μ), which are two electrical monopoles, with electrons tending to be more attracted to chlorine. So the bond H ─ Cℓ will have a partial negative charge on chlorine (δ-) and a partial positive charge on hydrogen (δ+). So this is a molecule with electronegativity difference and is polar:
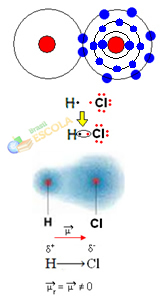
This shows us that electronegativity is a relative rather than an absolute quantity, as it is determined by taking into account comparisons of forces exerted by atoms in a covalent bond.
There are several ways to calculate electronegativity, but the most common is the electronegativity scale proposed by Pauling. Let's say we have a generic molecule A ─ B. Pauling proposed that the binding energy of this molecule, symbolized by D, would be given by the sum of the arithmetic mean of the binding energies (D) of the gas molecules of these two atoms, that is, A-A and B-B, with the square of the difference in electronegativities of each atom of that molecule (xTHE and xB):
Do not stop now... There's more after the advertising ;)
D(A-B) = [D(A-A) + D(B-B)]+k(xTHE - xB)2
The constant k in the formula above is equal to 96.5 kJ. mol-1. Pauling assigned an arbitrary value for the electronegativity of hydrogen, which was 2.1, and, in this way, it was possible to discover the electronegativity value of the other elements in relation to he.
Based on this method, the Pauling electronegativity values were given for the elements of the Periodic Table, with the exception of the noble gases.

Note that these values are a periodic property as they vary periodically as a function of the atomic numbers of the elements. See, for example, that the most electronegative elements are those in the upper right corner of the table, that is, fluorine (4.0) and oxygen (3.5), and the least electronegative are those in the lower left corner, which are francium (0.8) and cesium (0,8).
Based on this, it was even created a row of electronegativity of the most electronegative elements that tend to be worked the most:
F > O > N > Cℓ > Br > I > S > C > P > H
See the electronegativity values:
4,0 > 3,5 > 3,0 > 3,0 > 2,8 > 2,5 > 2,5 > 2,5 < 2,1
There is a kind of "trick" to decorate this row of electronegativity, which is given by the sentence below, in which the initial of each word corresponds to the symbol of the elements in question:
“Fhi Odo not have NO Clube, bri got IsOuch Çdying Pfor the Hhospital"
So we can say that electronegativity is a periodic property that increases from left to right and bottom to top on the Periodic Table.

This is because of the size of the atomic radius. The larger the radius of an atom, the further away the shared electrons are from its nucleus and, therefore, the weaker the attraction between them. The opposite is also true, the smaller the atomic radius, the closer the electrons will be to the nucleus and the greater the attraction between them. Thus, we can conclude the following:
Electronegativity increases with decreasing atomic radius.
By Jennifer Fogaça
Graduated in Chemistry