In this material, you will follow step-by-step resolutions and justifications for the responses of various exercises on chemical balance, which cover several topics in this important branch of Physical Chemistry.
1- Equilibrium constant in terms of concentration in mol/L
Example: (PUC-RS) An equilibrium involved in the formation of acid rain is represented by the equation:
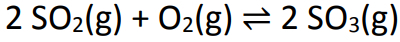
In a one liter container, 6 moles of sulfur dioxide and 5 moles of oxygen were mixed. After some time, the system reached equilibrium, and the number of moles of sulfur trioxide measured was 4. The approximate value of the equilibrium constant is:
a) 0.53
b) 0.66
c) 0.75
d) 1.33
e) 2.33
Right answer: Letter D
The exercise asks to calculate the equilibrium constant in terms of mol/L concentration. For this calculation to be performed, we must use equilibrium values for each participant in the reaction. The expression of Kc presents the result of multiplying the concentrations of the products divided by the product of the concentrations of the reagents:
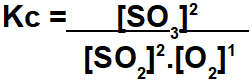
We must be very careful to determine the values of each participant in the balance, as the exercise will not always provide these data, as is the case in this example. So, we must follow the steps below:
Step 1: Assemble a table with known values.
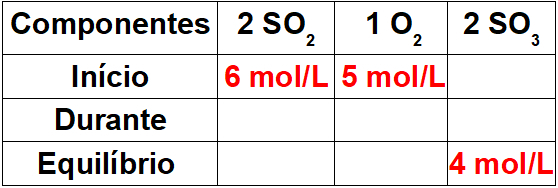
As this is the beginning of the reaction, the product will have a concentration equal to zero. As the equilibrium value in the product is always equal to the sum of onset and during, the value during the reaction will be 4 mol/L.

Step 2: Determine the values during the reaction.
To determine the values of the reagents during the reaction, it is enough to relate the known value for the product to the values of the reagents using the stoichiometric ratio. We have 4 mol/L of SO3 during the reaction for the proportion 2 in the balance. As the proportion of the OS2 is also 2, we will have 4mol/L during the process. to the O2, we will only have 2 mol/L, as its stoichiometric coefficient is 1.

To finalize the table, it is enough to subtract the start value by the during value, so that we will determine the equilibrium values for the reactants.

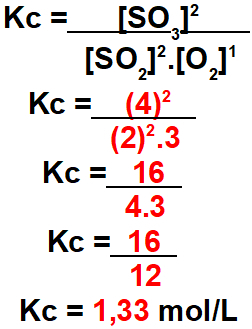
Step 3: Determine the value of Kc.
To determine the value of Kc, just use the values found in the equilibrium in the expression below:
2- Equilibrium constant in terms of partial pressure
Example: (SANTOS-SP) Observe the equilibrium equation below:
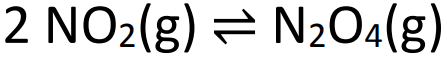
When the above equilibrium is reached, the pressure is 2 atm and there is 50% NO2 in volume. The value of the equilibrium constant in partial pressures (Kp) should be:
a) 0.2
b) 0.25
c) 1
d) 0.5
e) 0.75
Right answer: Letter C
The exercise indicates that the total system pressure at equilibrium is 2 atm and that there is 50% (mole fraction) of NO2. So, initially, we must determine the partial pressure for each gas at equilibrium by multiplying the total pressure by the molar fraction:
to NO2:
pNO2 = 0,5. 2
pNO2 = 1 atm
To the N2O4: as there are only two gases in the system, the percentage of N2O4 it will also be 50% to result in a total of 100%.
pN2O4 = 0,5. 2
pN2O4 = 1 atm
The equilibrium constant, in terms of partial pressures, is calculated by dividing the result of the multiplication of partial pressures of gaseous products by the product of reagent pressures gaseous. In this case, the expression of Kp will be:

3- Balance shift
Example: (PUCCAMP) The formation of stalactites, calcium carbonate deposits existing in caves close to limestone-rich regions, can be represented by the following reversible reaction:

Please observe the following conditions:
I. Constant water evaporation
II. Cold and damp air current
III. Temperature rise inside the cave
IV. Lowering the temperature inside the cave
Which of these conditions favor the formation of stalactites?
a) I and II
b) I and III
c) II and III
d) II and IV
e) III and IV
Right answer: Letter B
Stalactites are structures formed by calcium carbonate (CaCO3). The statement questions which of the indicated conditions favor the formation of stalactites. It is, therefore, an exercise about equilibrium shift, because the formation of CaCO3 occurs when the balance is shifted towards your direction (to the left).
I- True, because when it evaporates, the amount of water (present to the left of the balance) decreases. According to principle of Le Chatelier, when a participant's concentration decreases, the balance always shifts to their side.
II- False, since caves are cold and humid places, so the direct reaction of stalactites formation is exothermic. If a current of cold, humid air, which favors the exothermic process and increases the amount of water, entering the cave, the reaction will be shifted in the direct direction, not favoring the formation of stalactites.
III- True, as caves are cold and humid places and the direct reaction is exothermic, if the temperature in the cave increase, the reaction will be displaced in the indirect direction (endothermic), which will favor the formation of stalactites.
IV- False, as caves are cold and humid places and the direct reaction is exothermic, if the temperature in the cave decreases, the reaction will be shifted in the direct direction (exothermic), which will not favor the formation of stalactites.
See too:Chemical balance in caves
4- Ionization constant
Example: (UECE) The concentration [H+] of a 6×10 solution-7 mol/liter of acid H2S, with a Ki ionization constant of 10-7, it's the same as:
a) 5×10-7 mols/liter
b) 6×10-7 mols/liter
c) 3×10-6 mols/liter
d) 2×10-7 mols/liter
Right answer: Letter D
Since we only have one acid or one base, this is an exercise about ionization constant (Ki). So, to solve this kind of question, we must know the concentrations of the ions and the electrolyte (acid or base).
To start solving an exercise on the ionization constant, we must use the acid ionization equation (in the case of the exercise, H2S) or the base.
Do not stop now... There's more after the advertising ;)

According to the assembled equation, the concentration of H+ is the same as HS- in equilibrium due to the stoichiometric proportion. As we do not know these values, we will use x for both concentrations.
Note: we can use x for both concentrations because we are dealing with the product.
Step 1: Assembling the Ki expression.
The assembly of the expression of the ionization equilibrium constant follows the same principle of the constant in terms of concentration in mol/L.
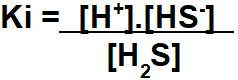
Step 2: Use the values provided by the exercise in the assembled Ki expression.

Step 3: Calculate the delta value.

Step 4: Calculate the possible x value for the delta found.

For x1
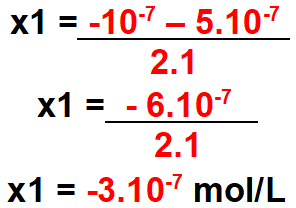
Note: the concentration cannot be negative. So this value is not valid.
For x2

5- Ostwald's law of dilution
Example: (ITA) In a 0.100 mol/L aqueous solution of a monocarboxylic acid at 25°C, the acid is 3.7% dissociated after equilibrium has been reached. Check the option that contains the correct value for this acid's dissociation constant at this temperature.
a) 1.4
b) 1.4×10-3
c) 1.4×10-4
d) 3.7×10-2
e) 3.7×10-4
Right answer: Letter C
Through Ostwald's law of dilution, we calculate the ionization constant (Ki) of a strong electrolyte (α is greater than 5%) using the formula:

To calculate the ionization constant of a weak electrolyte (α is less than 5%), we use the following formula:
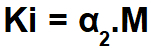
An exercise on Ostwald's law of dilution is easily recognized as it presents a concentration in mol/L (in this case 0.100 mol/L) of a single electrolyte (monocarboxylic acid), a dissociation percentage (α = 3.7%) or the dissociation or ionization constant (Ki).
As the acid is weak, so:

6- Chemical balance involving pH and pOH
Example: (PUC-MG) In three containers X, Y and Z are contained unknown basic solutions with a concentration of 0.1 mol/L. By measuring the pH of the three solutions with universal indicator paper, the following values were obtained, respectively: pH = 8, pH = 10 and pH = 13. Tick the CORRECT statement:
a) The concentration of OH- of base Z is equal to 10-13 mol/L.
b) Kb from base X is greater than Kb from base Y.
c) Base Y conducts electrical current better than base Z.
d) Base X is completely ionized.
e) In bottle Z, a strong base is contained.
Right answer: Letter e
To start solving this exercise, it is necessary to remember some important points:
First: pH + pOH = 14
Second: the higher the pH, in relation to the value 7, the more basic the solution will be. The more basic the solution, the greater the concentration of hydroxide anions [OH-].
Third: [OH-] = 10-pOH
Room: the smaller the pOH, the larger the Kb, that is, the more ionized or dissociated the base will be.
So, based on this knowledge, just follow the step by step below to resolve the issue:
Step 1: Determine the pOH of each solution.
For solution X:
pH + pOH = 14
8 + pOH = 14
pOH = 14 - 8
pOH = 6
For solution Y:
pH + pOH = 14
10+ pOH = 14
pOH = 14 - 10
pOH = 4
For solution Z:
pH + pOH = 14
13 + pOH = 14
pOH = 14 - 13
pOH = 1
Step 2: To judge alternative A, we must determine the hydroxide concentration for solution Z.
[oh-] = 10-pOH
[oh-] = 10-1 mol/L,
Soon, the alternative A is false.
Step 3: Compare the base X Kb with the base Y.
The base X Kb is smaller than the base Y Kb because its pOH is larger. Soon, the alternative B is false.
Step 4: Associate pOH with strength and dissociation.
The conduction of electric current occurs best in solutions that have a strong electrolyte with a higher pOH. Base Y does not conduct electrical current better than base Z because its pOH is lower, so fewer ions are released. So, the alternative C is false.
Step 5: Relate pOH with dissociation.
The smaller the pOH, the more dissociated the base. As the solution with the highest pOH is in container X, it contains the least dissociated solution. Therefore, the alternative D is false.
See too: The pH of the mouth and tooth decay
7- Buffer solution
Example: (UFES) The pH of human blood is maintained within a narrow range (7.35 - 7.45) by different buffer systems. Point out the only alternative that can represent one of these buffer systems:
a) CH3COOH / NaCl
b) HCl / NaCl
c) H3DUST4 / NaNO3
d) KOH / KCl
e) H2CO3 / NaHCO3
The answer to this question is the alternative E, because this is an exercise in buffer solution or buffer system. This solution refers to a chemical balance formed by a mixture of two solutions: an acid (in exercise, the H2CO3) or weak base and a salt that has the same acid component (in exercise, NaHCO3) or the base.
a- False, because it is a mixture formed by a weak acid and a salt that has no acid component.
b- False, because it is a mixture formed by a strong acid, since HCl is one of the three strong hydracids (the others are HBr and HI).
c- False, because it is a mixture formed by a moderate acid and a salt that has no acid component.
d- False, because it is a mixture formed by a strong base (it has an element of the alkali metal family).
See too: Buffer solution in human blood
By Me. Diogo Lopes Dias