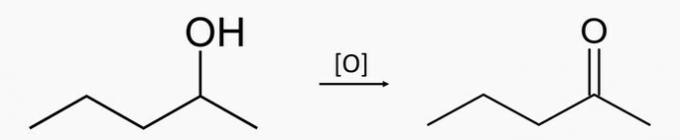
You Hydrocarbons are organic compounds characterized by having only carbon and hydrogen atoms. As shown in the text “IUPAC Nomenclature”, the nomenclature of the compounds of this function, which have no branches and are open-chain, follows the following basic rule:
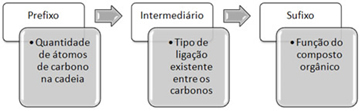
Hydrocarbons can be divided into alkanes, alkenes and alkynes. The nomenclature of each of these groups, without the presence of ramifications, can be seen in more detail in the texts: Alkanes Nomenclature, Alkenes Nomenclature and Alkyne Nomenclature .
But, basically, the difference between them is in the intermediary, that is, in the type of connection, which is as follows:
- Range: Link simple: intermediary an
- Alkene: Link pair: intermediary en
- Alkyne: Link triple: intermediary in
With this information in mind, let's now look at the naming rules for these hydrocarbons when they have branches and when they are cyclic or aromatic:
- Branched hydrocarbons:
First, it is necessary choose the main chain, which should have the following main characteristics:
1- Encompass the greatest number of unsaturations;
2- Have the longest sequence of carbon atoms bonded together.
Example:
Wrong: Correct:
H3C — CH2 - CH - CH2 — CH2 — CH3 H3C — CH2 —CH — CH2— CH2 — CH3
│ │
CH2 CH2
│ │
CH2CH2
│ │
CH3 CH3
The first one is wrong because it contains only 6 carbon atoms, while the second one, the main chain has 7 carbons.
If you happen to have more than one possibility of a chain with the same amount of carbons, you should choose the one that has greater number of branches. See the examples below:
CH3 CH3 CH3
│ │ │
H3Ç —CH2 —CH —CH —CH3 H3C — CH2 — CH —CH —CH3 H3C — CH2 — CH —CH — CH3
│ ││
H3C — CH2 H3Ç - CH2H3Ç —CH2
│ │ │
CH3 CH3 CH3
Do not stop now... There's more after the advertising ;)
Note that in all three cases the chains chosen as main (in red) have 5 carbons. The first structure has 2 branches (in black), the second has 3 branches and the third chain also has 3 branches. Therefore, the main string chosen correctly is the second or third (which will actually lead to the same naming).
After choosing the main chain, it is necessary number it, as it will be necessary to indicate which carbon the branch is coming from. To learn the nomenclature of the branches, read the text “Branch naming”.
Thus, the nomenclature of branched open chain hydrocarbons follows the following order:
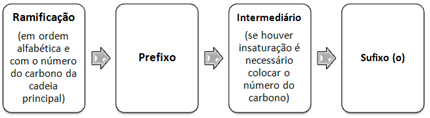
Now see the following examples:
H3C — CH2 — 4CH —3CH2 — 2CH2 — 1CH3 : 4-ethylheptane
│
5CH2
│
6CH2
│
7CH3
1CH3
│
H3C — CH2 — 3 CH — 2CH — CH3: 3-ethyl-2,4-dimethylpentane
│
H35Ç -4CH2
│
CH3
H38Ç - 7CH2 – 6CH2 – 5CH2 – 4CH - 3CH = 2CH -1CH3: 4-s-butyl-oct-2-ene
│
CH3 — CH2 — CH
│
CH3
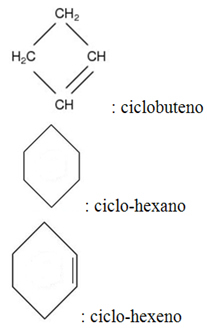
- Cyclic hydrocarbons:
The only difference between open-chain and cyclic-chain hydrocarbons is the presence of the prefix "cycle".
Examples:
- Aromatic Hydrocarbons:
There is no general rule for naming Aromatic Hydrocarbons, in general, these compounds have a particular nomenclature.
When its main chain has only one benzene ring, it is called benzene and may have one or more substituent groups. Let's look at the structural formulas for the most common aromatics:

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Nomenclature of cyclic and branched hydrocarbons"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/nomenclatura-hidrocarbonetos-ciclicos-ramificados.htm. Accessed on June 27, 2021.
Alkanes nomenclature, hydrocarbon function, carbon valences, International Union of Pure and Applied Chemistry, IUPAC, saturated aliphatic hydrocarbons, single bonds, compounds Organic.