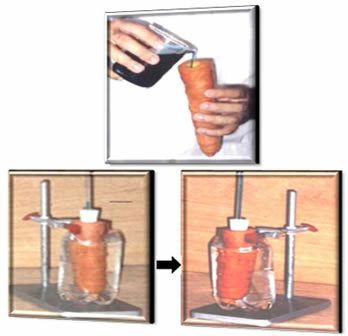
THE Osmotic Pressure can be briefly defined as the pressure necessary to prevent osmosis from occurring spontaneously in a system, that is, that the solvent from a more dilute solution passes to a more concentrated one through a membrane semipermeable.
But how to osmoscopy is joint ownership, this factor depends on the amount of dissolved particles, which is different for molecular and ionic solutions. Therefore, the way to calculate the osmotic pressure (π) is also different for these two cases.
Molecular solutions are those in which the solute does not ionize in water, that is, it does not form ions, but its molecules simply separate from each other and are dissolved in the solution. In these cases, the calculation of the osmotic pressure can be done by the following mathematical expression:
π = M. A. T
M = solution molarity (mol/L);
R = universal constant of perfect gases, which equals 0.082 atm. L. mol-1. K-1 or 62.3 mm Hg L. mol-1. K-1;
T = absolute temperature, given in Kelvin.
This expression was proposed by scientist Jacobus Henricus Van 't Hoff Junior after he observed that the osmotic pressure has a behavior very similar to that shown by the ideal gas. From this, Van 't Hoff Júnior proposed a way to determine the osmotic pressure (π) through the ideal gas equation (PV = nRT).
For example, if we mix sugar with water, we will have a molecular solution, because sugar (sucrose) is a molecular compound whose formula is C12H22O11. Its molecules are simply separated by water, breaking away from each other, remaining whole and undivided.
Ç12H22O11(s)→Ç12H22O11(aq)
The amount of molecules present is calculated through the relationship between the number of moles and the number of Avogadro, as shown below:
1 mole of C12H22O11→(s)1 mole ofÇ12H22O11(aq)
6,0. 1023 molecules→6,0. 1023 molecules
Note that the amount of dissolved molecules remains the same as before they were dissolved in water.
Thus, if we consider a 1.0 mol/L sucrose solution at a temperature of 0°C (273 K), the pressure that must be exerted to prevent osmosis of this solution should be equal to:
π = M. A. T
π = (1.0 mol/L). (0.082 atm. L. mol-1. K-1). (273K)
π ≈ 22.4 atm
But if the solution is ionic, the amount of particles dissolved in the solution will not be the same as the amount placed at the beginning, as there will be an ionic dissociation or ionization of the solute with formation of ions.
Do not stop now... There's more after the advertising ;)
For example, imagine that 1.0 mol of HCℓ is dissolved in 1 L of solvent, will we have a concentration of 1 mol/L like what happened with sugar? No, because HCℓ undergoes ionization in water as follows:
HCℓ → H+(here) + Cℓ-(here)
↓ ↓ ↓
1 mole 1 mol 1 mol
1 mol/L 2 mol/L
Note that 1.0 mol of solute formed 2.0 mol of solute, which affects the solution concentration and, consequently, the value of the osmotic pressure.
See another example:
FeBr3 → Fe3+ + 3 Br-
↓ ↓ ↓
1 mole 1 mol 3 mol
1 mol/L 4 mol/L
Did you see? The concentration of ionic solutions varies from solute to solute, as the amount of ions generated is different. Thus, when calculating the osmotic pressure of ionic solutions, this amount needs to be taken into account.
For this reason, you must introduce a correction factor for each ionic solution, which is called the Van’t Hoff factor (in honor of its creator) and is symbolized by the letter “i”. The Van’t Hoff factor (i) of the HCℓ solution mentioned is 2 and that of the FeBr solution3 é 4.
The mathematical expression used to calculate the osmotic pressure of ionic solutions is the same as that used for molecular solutions plus the Van't Hoff factor:
π = M. A. T. i
See this calculation for the mentioned HCℓ and FeBr solutions3 at the same temperature of 0ºC and considering that both solutions have a concentration of 1.0 mol/L.
HCℓ:
π = M. A. T. i
π = (1.0 mol/L). (0.082 atm. L. mol-1. K-1). (273K). (2)
π ≈ 44.8 atm
FeBr3:
π = M. A. T. i
π = (1.0 mol/L). (0.082 atm. L. mol-1. K-1). (273K). (4)
π ≈ 89.6 atm
These calculations show that, the greater the concentration of the solution, the greater the osmotic pressure.This makes sense because the tendency for osmosis to occur will be greater and we will also need to apply greater pressure to be able to stop it.
By Jennifer Fogaça
Graduated in Chemistry
Chemistry

Colligative properties, tonoscopy, ebullioscopy, cryoscopy, osmoscopy, colligative effects, reduction of chemical potential of solvent, boiling temperature, melting point drop, osmotic pressure, non-volatile solute, solute, solvent, tempe