In sports activities, in hospitals and in our daily lives, the use of hot and cold instant compresses is very common. In both cases, certain substances react with water. For example, in the case of the cold compress, it contains two capsules separating the water from the NH4AT THE3, which, when dissolved in water, absorbs heat and produces instant cold. In the case of hot compresses, CaCl dissolves in water2 or the MgSO4, which release energy producing heat.
But why certain solutions give off heat, being exothermic; and others absorb, being endothermic?
Well, to understand this issue we have to study the enthalpy variation (ΔH) of solutions, which is made up of two steps:
(1st) Reticular enthalpy (ΔHret): when a solute dissolves in water, the first step is to separate its ions which are in a crystalline lattice. To break the bonds between the ions it is necessary to supply energy to the system. So this first process is endothermic, as it absorbs energy; being yours positive enthalpy (ΔH > 0).
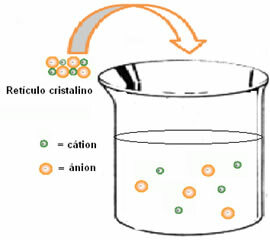
(2nd) Enthalpy of hydration (ΔHhid): after the ions separate, they are enveloped by the solvent molecules. In the case of water, it is the solvent and we say that hydration is taking place. The dipoles of water are respectively attracted by the oppositely charged ions; thus, for this interaction to take place, the release of energy is necessary. Thus, in hydration the enthalpy will be negative (ΔH < 0), because the process is exothermic.
Do not stop now... There's more after the advertising ;)
The figure below shows how hydration occurs, in which there is ion-dipole interaction, that is, attraction between the charges of the separated ions and the water dipole:
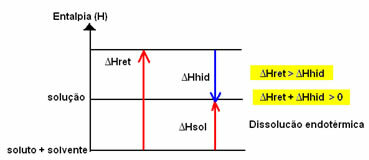
The enthalpy change of the solution (ΔHSun) will be determined by the sum of these two enthalpies. If the result is positive, it means that the reticular enthalpy is higher, so the dissolution enthalpy will indicate that the process is endothermic.
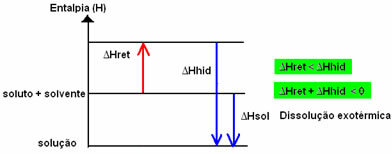
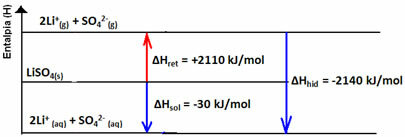
The enthalpy diagram of an endothermic dissolution is shown below:
This is indicated by the case of potassium iodide dissolution shown below:
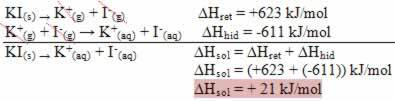
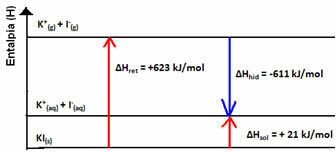
Your enthalpy diagram will be represented by:
If the result is positive, the enthalpy of hydration will be greater than the reticular one and the process is exothermic. The enthalpy diagrams of exothermic dissolutions are represented as shown in the following example:
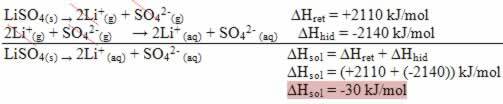
Note your enthalpy diagram below:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Solution Enthalpy Variation"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/variacao-entalpia-solucao.htm. Accessed on June 28, 2021.