For a combustion reaction and a fire to occur, three factors are needed: fuel, oxidizer and chain reaction.
1- Fuel:
Fuel is any material that can be oxidized. They can be solid (paper, wood, cotton), liquid (alcohol, gasoline, ether, fuel oil) or gaseous (hydrogen gas, acetylene, LPG (Liquefied Petroleum Gas)).

Among liquid fuels, we have volatiles, which are those that release vapors at room temperature, such as alcohol, gasoline and ether; and there are also non-volatile ones, which practically do not release vapors, such as paint, fuel oil, grease, among others. Volatiles pose greater risk.
2- Oxidising:
The oxidizer is oxygen gas (O2), which comes in contact with the fuel and reacts. It is indispensable for combustion to occur and this can be seen through a simple and well-known experiment: if If you place a glass over a lighted candle, the flame will go out with time, as all the oxygen has been consumed and the reaction ceases.
3- Chain reaction:
Heat provides the energy needed for the reaction to continue. For example, grass is a fuel and it is in contact with oxygen in the air, but for its burning to occur it is an ignition, or activation energy, which is supplied, for example, by a spark, as when someone throws a lit cigarette. Then combustion begins, releasing heat that provides the minimum energy needed for the chain reaction to continue.
Thus, combustion is represented by a diagram called the fire triangle:
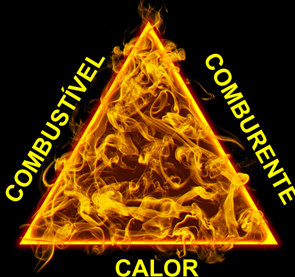
So, to fight a fire, we have to remove one of these three factors. See how:
1-Eliminating heat:
One of the main ways to fight a fire is through cooling, lowering the temperature. This method is ideal for class A fires, which occur with solid fuels that burn at the surface and at its depth, leaving residues (ashes).
Do not stop now... There's more after the advertising ;)

To eliminate heat, use extinguishers appropriate for each type of fire. But it is necessary to know exactly which extinguisher to use for each type of fire, otherwise it can make the situation worse. For example, let's say the fire is occurring in electrical equipment that poses a shock hazard (class C fire), in this case, a water extinguisher would not be indicated, but a chemical powder extinguisher dry.
For more details, read the text. Classification of fire extinguishers.
2-Eliminating the fuel:
This is done by removing the material from the site. For example, let's say a hydrogen gas inlet is open, we can then close it, removing the fuel that is being burned.
This method is suitable for class B and C fires, which are, respectively, fires with liquids that burn on the surface and leave residues and fire with electrical equipment, as already mentioned.
3- Eliminating the oxidizer:
We can prevent the fuel from coming into contact with oxygen by smothering it with a cover.
For example, if the cooking oil has started to catch on fire, you should never throw water in, because if you do, there will be some kind of explosion and you will get hurt. This is because oil is less dense than water. Thus, when splashing water, it would tend to sink, passing through the hot oil. Since the temperature is above the boiling point of water, it would immediately change from a liquid to a gas, forming bubbles of steam that would rise rapidly through the hot fat and bounce extremely violently into the air. These bubbles would carry oil droplets that could burn the skin if they fell on it.
The correct way would be do not touch the pan, turn off the heat and place a damp cloth over the pan to prevent oxygen from reaching the flames and, consequently, they will go out.

By Jennifer Fogaça
Graduated in Chemistry


