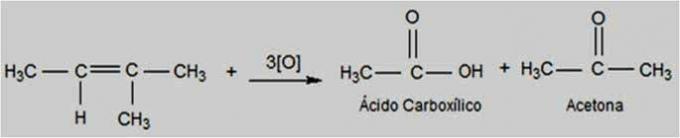
A solution is a homogeneous mixture of two or more substances.. As, for example, a solution of salt (solute) dissolved in water (solvent).

Especially in chemical laboratories and industries, this process is very important, because the chemist needs to prepare solutions with known concentrations. Furthermore, in experimental activities, solutions with very low concentrations are used, so a sample of the concentrated solution is diluted to the desired concentration.
On a daily basis, several times, without even realizing it, we carry out the process of diluting solutions. For example, the packaging of cleaning and domestic hygiene products, such as disinfectants, advises that they be diluted before use. Some manufacturers suggest on product labels that it be diluted with water in a proportion of 1 to 3, that is, for each part of the product, 3 parts of water must be added. This is done because the product is very concentrated and strong and can damage the place where it will be applied if it is not diluted in the right way. On the other hand, if you dilute it more than it should, you can lose money, because the product will not achieve the desired result.

Another example is when we make juices. The labels of many juice concentrates indicate that a glass of juice should be diluted or mixed with 5 glasses of water. Thus, the juice becomes “weaker”, that is, less concentrated.
Do not stop now... There's more after the advertising ;)
Imagine that you have diluted such a juice in 3 L of water. If the initial juice concentration was 40g/L, it means that it had a mass of 40g for each liter of solvent. But since we will have 3 L, the mass will be divided by 3 and the concentration will then be approximately 13, 33 g/L, or 13 grams for each liter of solution. However, in the entire solution, the mass of the 40g solute still remains.
The calculation of this new concentration can be done as follows:
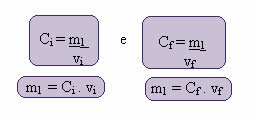
Where the indexes i and f represent, respectively, the initial and final values. Since the value of m1 has not changed, we can equalize the equations:
Çi. vi = Cf. vf
Replacing the values we have, according to the previous example, note:
Initial solution:
Çi: 40g/L
m1: 40g
vi: 1L
Final solution:
Çf: ?
m1: 40g
vf: 3L
Çi. vi = Cf. vf
(40 g/L). (1 L) = Cf. 3L
Çf = 40 g/L
3
Çf = 13.333 g/L
The same reasoning is also valid for the molar concentration (M) and for the percentage by mass of solute or titer (T):
Mi. vi = Mf. vf and Ti. vi = Tf. vf
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Dilution of Solutions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/diluicao-solucoes.htm. Accessed on June 27, 2021.