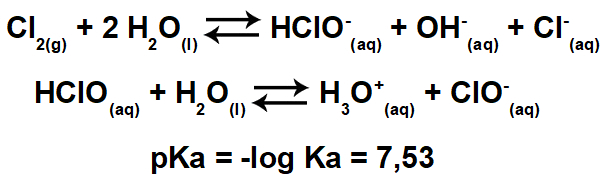The text Titration showed how this volumetric analysis technique is performed, whose main objective is identify the concentration of a solution through its reaction with another solution of known concentration.
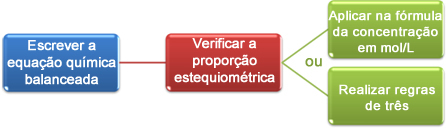
Now, we will see how to use the data obtained in the titration to arrive at the desired concentration, which can be an acid or a base in solution. To do this, there are basically three steps:
Let's look at an example:
Let's say a chemist had a solution of acetic acid (vinegar (CH3COOH(here))) and wanted to find out its concentration in mol/L. Then, he placed 20.0 mL of vinegar (titrated) in an Erlenmeyer flask and added the phenolphthalein indicator. Then he filled a 100 mL burette with sodium hydroxide (NaOH) of known concentration (titrant) equal to 1.0 mol/L. Finally, the chemist performed the titration and noticed that the color change (turning point - when he stopped the titration) occurred when 24 mL of NaOH was consumed.

Based on this experiment, he obtained the following data:
MCH3COOH= ?
VCH3COOH = 20 mL = 0.02 L
MNaOH = 24 mL = 0.024 L
VNaOH = 1.0 mol/L
Where M = concentration in mol/L and V = volume in L.
To find out the concentration of acetic acid, we must first know how to write the chemical equation that represents the properly balanced neutralization reaction that occurred. In this case, the reaction is as follows:
1 CH3COOH(here) + 1 NaOH(here) → 1 NaC2H3O2(aq) + 1 hour2O(ℓ)
This part is important to see the stoichiometric ratio at which the reactants react. Note that the ratio is 1:1, that is, for every mole of acetic acid, 1 mole of sodium hydroxide is needed.
Now we can proceed with the calculations in two ways:
(1st) Through the formula: M1. V1 = M2. V2
Since the stoichiometric ratio is 1:1, we have to: noCH3COOH = nNaOH .
Do not stop now... There's more after the advertising ;)
Being M = n/V → n = M. V. Thus, we arrive at the above list, which can, in this case, be written like this: MCH3COOH. CH3COOH = MNaOH. VNaOH
So, just replace the values of this formula:
MCH3COOH. V CH3COOH = MNaOH. VNaOH
MCH3COOH. 0.02 L = 1.0 mol/L. 0.024 L
MCH3COOH = 0.024 mol
0.02 L
MCH3COOH = 1.2 mol/L
Therefore, the initial concentration of the acetic acid solution, our title, was 1.2 mol/L.
Important note: If the stoichiometric ratio were not 1:1, it would be enough to multiply the concentration in mol/L (M) of the substances by their respective coefficients. For example, if the ratio were 1: 2, we would have the following:
M1. V1 = 2. M2. V2
But here's another way to perform these calculations:
(2nd) Through rules of three:
1 CH3COOH(here) + 1 NaOH(here) → 1 CH3COONa(here) + 1 hour2O(ℓ)
1 mol 1 mol 1 mol 1 mol
1. 60g 1. 40 g 1. 82 g 1. 18 g
These masses are the calculated molecular masses for each substance.
* Knowing that the spent volume of the 1.0 mol/L NaOH solution(here) was 24 mL, we can first find out the amount of matter (mol) of NaOH that reacted:
1 mol of NaOH → 1.0 L
1 mol of NaOH 1000 ml
x 24 ml
x = 0.024 mol of NaOH
* Since the ratio is 1:1, the amount of matter (mol) of acetic acid should be the same as NaOH: 0.024 mol.
Observation: If the stoichiometric ratio were different then we would take that into account in this part. For example, if it were 1:3 and the amount of matter of one chemical reactive species was equal to 0.024 mol, then that of the other substance would be triple: 0.072.
* Now we calculate:
20 ml of vinegar 0.024 mol of acetic acid
1000 ml of vinegar and
y = 1.2 mol
That is, there is 1.2 mol/L, which is the same value we got in the previous method.
By Jennifer Fogaça
Graduated in Chemistry