An oxidation-reduction reaction is characterized as a simultaneous process of loss and gain of electrons, as the electrons lost by an atom, ion or molecule are immediately received by others.
To understand, see an example:
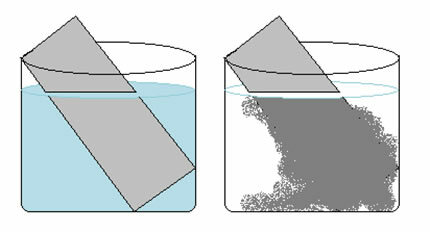
A copper sulfate solution (CuSO4(aq)) is blue due to the presence of Cu ion2+ dissolved in it. If we put a metallic zinc plate (Zn(s)) in this solution, over time we may notice two changes: the color of the solution will become colorless and a metallic copper deposit will appear on the zinc plate.
Therefore, the reaction that occurs in this case is as follows:
Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
or
Zn(s) + Cu2+(here) + OS42-(here) → Cu(s) + Zn2+(here) + OS42-(here)
or yet
Zn(s) + Cu2+(here) → Cu(s) + Zn2+(here)
Note that there was a transfer of electrons from zinc to copper. Analyzing in isolation the transformation that occurred in each of these elements, we have:
- Zn(s) → Zn2+(here)
Zinc lost 2 electrons going from metallic zinc to cation. In that case, the zinc has undergone an oxidation.
- Ass2+(here) → Cu(s)
With copper, the opposite happened, it gained 2 electrons, passing from copper II cation to metallic copper. Copper has been reduced.
This explains the two changes observed, as the solution became colorless because the copper ions were transformed into metallic copper, which was deposited on the zinc plate.
Do not stop now... There's more after the advertising ;)
Since there was a simultaneous loss and gain of electrons, this reaction is an example of an redox reaction, and through it we can establish the following concepts that are repeated for all other reactions of this type:

The most reactive metal undergoes oxidationThus, in the proposed example, zinc is more reactive than copper.
Another redox reaction that can be cited occurs when we put magnesium or aluminum into a hydrochloric acid solution. In these reactions, hydrogen from hydrochloric acid receives 3 electrons from aluminum (or 2 electrons from magnesium), passing it from H cation+ for hydrogen gas (H2), while the metal becomes the cation:
2 Al(s) + 6 H+(here) → 2 Al3+(here) + 3H2 (g)
mg(s) + 2 H+(here) → Mg2+(here) + H2 (g)
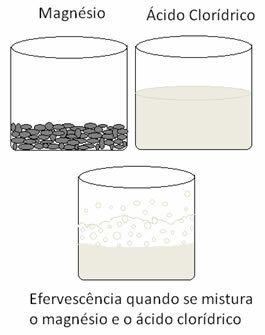
Metals undergo oxidation and hydrogen undergoes reduction. Below is a figure that shows that adding magnesium to hydrochloric acid causes a effervescence, which is due to the release of hydrogen gas, and the magnesium disappears, as it is consumed.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Oxidation Reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-oxirreducao.htm. Accessed on June 28, 2021.
Chemistry
Air bag operation, device designed to protect drivers, electrical impulse, chemical decomposition reaction, collision, chemical mixture of sodium azide, sensors located on car bumper, alkali silicate, gas nitrogen.
Chemistry

Photosensitive lenses, oxidation-reduction reactions, loss or gain of electrons, photosynthetic lenses in sunglasses, composition of photochromatic glass, tetrahedral oxygen atoms, crystal structure of silver chloride, ultraviolet light, silver metal