In 1874, the Dutch chemist Jacobus Henrique van’t Hoff (1852-1911) and the French chemist Joseph Achille le Bel (1847-1930), through mathematical theories, independently suggested the existence of carbons asymmetric.
At the time, they were heavily criticized, most notably van’t Hoff, by the famous German chemist Adolph Wilhelm Kolbe (1818-1884). However, Kolbe was wrong, today we know that there is an asymmetric carbon, so much so that, in 1901, van’t Hoff was the first chemist to receive the Nobel Prize.
van’t Hoff (left) and Kolbe (right)
One of the necessary conditions for the molecule to have optical activity is that it be asymmetric. Also, one way to check if it is asymmetric is to study its structure in detail to see if it has at least one asymmetric carbon.
What is an asymmetric molecule and an asymmetric carbon?
Something symmetric is something that has at least one plane of symmetry. For example, if we cut a tennis racket in half, the two resulting parts will be exactly the same. Furthermore, if we place them in front of a flat mirror, they will produce an identical image.
Structures that do not support a plane of symmetry are called asymmetric. An example is our hand, because if we place it in front of a mirror, it will produce a different image of itself. If we put the right hand, the image will be the left hand and vice versa. Another important point is that they are not overlapping.
Do not stop now... There's more after the advertising ;)
That's why asymmetric carbon is also called chiral carbon, where this word originates from khéir which in Greek means hand.
An example of an asymmetric molecule is thalidomide, whose structure is shown below:

The dot located with an asterisk (*) corresponds to an asymmetric carbon, as it it has four covalent bonds that are made with different atoms or groups of atoms.
Below is a carbon with four different ligands in front of a mirror. Note that the image cannot be superimposed over the original structure:

The two molecules obtained above are optical isomers or enantiomers.
Returning to the case of thalidomide, due to the fact that it has asymmetric carbon, the result is that the molecule starts to have a non-superimposable image, which corresponds to another substance. So we have two isomers of thalidomide with extremely different properties.
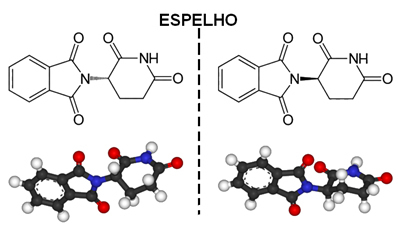
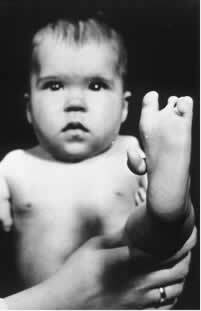
One of these (R) isomers has sedative properties. Therefore, in the late 1950s, it began to be used as a tranquilizer and sleep aid for pregnant women. This triggered a tragedy, as its (S) enantiomers were also mixed with the drug. This isomer, in turn, is teratogenic and has led many pregnant women to have their babies with atrophied hands, legs, arms and feet.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Asymmetric or Chiral Carbon"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/carbono-assimetrico-ou-quiral.htm. Accessed on June 27, 2021.