Silicones are polymers composed of intercalated silicon and oxygen, which also contain organic groups in their structure.
Silicon was invented in 1943, follow the reactions of the manufacturing process:
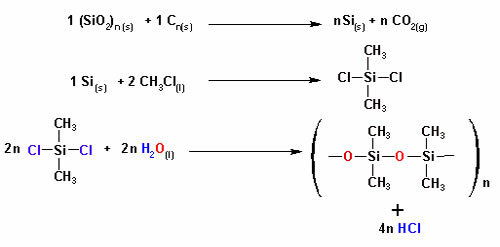
Note that the sand (SiO2) mixed with carbon gives rise to the final product, silicone. The respective chain has, in its linear chain, alternating silicon and oxygen atoms. The amount of organic branches - CH3 can vary in the molecule, hence the explanation for the variety of silicones on the market.
Silicone can range from a viscous liquid to a solid, similar to rubber. This variation in physical form depends on the size of the molecule, let's see:
- Silicone formed by smaller molecules has an oily aspect and is therefore used as a waterproofing surface, lubricating grease, polishing wax, etc. It is in liquid form and can be applied to enhance car bumpers and plastic panels.
- Intermediate molecules give rise to more pasty silicones, used in the manufacture of adhesives and sealants, such as silicone glues, used in the assembly and fixation of aquariums and windows glass.
- As the organic part of the silicone molecule gets larger, the bonds cross and the silicone takes on the appearance of an elastomer, better known as silicone rubber. Thus, it becomes highly resistant and is therefore used in industrial equipment and car parts, among others.
For those who associate the word silicone only with the female vanity of getting bigger breasts (silicone prostheses), we present here is another application of this polymer in the female universe: silicone is used in cosmetic formulations, such as in lipsticks.
Do not stop now... There's more after the advertising ;)
By Líria Alves
Graduated in Chemistry
Brazil School Team
See more! Breast silicone
Polymers - Organic chemistry - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Obtaining and using silicone"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/obtencao-uso-silicone.htm. Accessed on June 28, 2021.



