Polarized light was first observed in 1808 by Malus and Huygens, when observing a beam of light passing through the Icelandic spar, a transparent crystal of a variety of carbonate. calcium.

In 1812, Jean-Baptiste Biot observed that the polarized light beam was rotated, in some crystals, to the right and, in others, to the left. One important observation he made was that it wasn't just solid substances, or pure liquids that rotated the polarized light beam, but even aqueous solutions of certain organic substances had this property. This indicated that the phenomenon observed was due to the structure of the molecule itself.
Biot invented a device to observe the phenomenon of deviation from the plane of polarized light, which became known as polarimeter. In 1842 it was perfected by Ventzke, who adapted a Nicol prism to the apparatus, and years later Mitscherlich introduced the use of monochromatic light in observations.
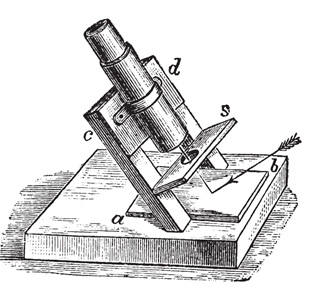
But it was only in 1846 that this phenomenon was explained, through studies of
Louis Pasteur, who was a student of Biot. During the process of fermentation of grape juice, for the production of wine, two acids are formed: tartaric acid and racemic acid.
Stamp printed by the Central African Republic shows Louis Pasteur (1822-1895), Chemist and Microbiologist, circa 1985*
Do not stop now... There's more after the advertising ;)
These two acids had the same molecular formula and the same properties, however, they behaved differently when subjected to polarized light beam. It was already known that the tartaric acid was optically active, rotating the polarized light plane to the right. Already the salts of racemic acid were inactive under polarized light.
Pasteur found that whereas tartaric acid was composed of only one type of molecule, racemic acid had two types. Carefully studying the salts that formed both acids, Pasteur found that the tartaric acid crystals were asymmetrical and the racemic acid crystals too. However, some crystals of the latter had a different face to the right and others to the left.
He carefully separated these crystals and dissolved them separately in water. After reviewing these solutions, he found that both were optically active. Therefore, the racemic acid was not pure, in fact, it was composed of half a type of dextrorotatory tartaric acid (which deviates from the plan of right polarization) and the other half of the levorotary tartaric acid type (which shifts the plane of polarization to the left).Since these two types caused a deviation of the same value, but with the opposite direction, one ended up canceling the other and the substance became optically inactive.
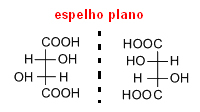
Thus, when a molecule has asymmetric carbons, as in the case of tartaric acid, it gives rise to two optical isomers, of the same molecular formula, but with different optical activities.
* Image credits: rook76 and Shutterstock.com
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "History of Optical Isomerism"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/historia-isomeria-Optica.htm. Accessed on June 28, 2021.