If it were possible to visualize the structure of a metal very clearly, we would see it as in the image above. The atomic structure of metals is Crystalline, which consists of metal cations surrounded by electrons.
The crystalline lattices present in metals can be represented as follows: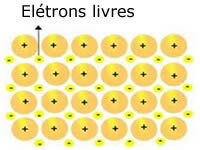
Representation of metallic sodium (Na)
The crystalline lattices of metals are formed by a group of fixed cations.
Each Na+ cation is surrounded by electrons, but these are delocalized, that is, they are not attracted to any nucleus.
As there is no attraction between negative charges (electron) and the positive nucleus (cation), the free electrons end up occupying the entire crystalline lattice of the metal. The freedom that electrons have to move causes them to form an electronic cloud.
The ability of metals to conduct electricity is explained by the presence of this cloud. Electric current results from the contact of free electrons with other metals.
In the composition of any atom, including metals such as Sodium (Na), Gold Au, Copper (Cu), there is a valence layer. Electrons move freely through this layer maintaining the electromagnetic attraction for cations. This structural property allows the formation of metallic molecules and, consequently, of the metals themselves.
By Líria Alves
Graduated in Chemistry
Do not stop now... There's more after the advertising ;)
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "How is the metallic bond formed?"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/como-se-forma-ligacao-metalica.htm. Accessed on June 28, 2021.