At aces or monosaccharides are compounds that belong to the class of carbohydrates, also called carbohydrates. This class is characterized by having mixed functions in its structure, with several compounds belonging to the alcohol group and one group being a ketone or an aldehyde.
What makes oses different from other carbohydrates is the fact that they do not hydrolyze.
As they have many asymmetric carbons, oses have many optical isomers. Remember that isomerism is a phenomenon that occurs when two or more different compounds have the same molecular formula, but different structural formulas.
For example, glucose and fructose are oses that have the same molecular formula: C6H12O6. The difference between these two groups is that the functional groups of these two substances are different. As you can see below, glucose is from the aldehyde group, so it is considered an aldose; on the other hand, fructose belongs to the group of ketones, being designated as a ketosis.

Glucose and fructose are isomeric compounds.
Optical isomerism denominates right-handed (D) the isomer that deflects polarized light to the right; and as levogiro (L), the isomer that deflects polarized light to the left plane.
Do not stop now... There's more after the advertising ;)
Applying this concept to the isomers of oses, oses are considered to be derived from glyceric aldehyde. This compound has two active optical isomers, as it has an asymmetric carbon:
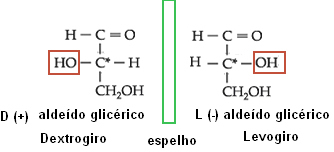
Isomerism of glyceric aldehyde.
Thus, for all oses that have the OH group on the right side of the second carbon, counting from the bottom to the top, the compound is considered to be in the D series. However, if the OH group is on the left side, it will be from the L series. The positive and negative signs serve to indicate whether the compound is right-handed or left-handed, respectively. Below are some examples:
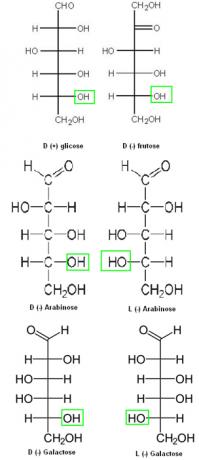
Examples of right-hand and left-handed series from the oses group.
Note that D (+) glucose, D (-) fructose, and D (-) arabinose differ only at carbons 1 and 2 (from top to bottom). When this happens, we say that these compounds are epimers.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Structure and Configuration of Oses"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/estrutura-configuracao-das-oses.htm. Accessed on June 28, 2021.