*Theory of Arrhenius:
Based on his experiments with electrical conductivity in aqueous media, the chemical, physical and mathematical Swedish Svante August Arrhenius (1859-1927) proposed, in 1884, the following concepts to define acids and bases:

So, generically, we have:
H+ + H2O → H3O+
Examples:
HCl + H2O → H3O++ Cl-
HNO3+ H2O → H3O+ + NO3-
H2ONLY4+ 2H2O → 2H3O+ + OS42-

Examples:
NaOH → Na + + OH-
Ca(OH)2 →Ca2+ + 2 OH-
*Brönsted-Lowry theory:
Independently, the Danish Johannes Nicolaus Brönsted (1879-1947) and the English Thomas Martin Lowry (1874-1936), proposed in the same year another acid-base theory known as the Brönsted-Lowry Theory, which says the Following:

In this case, the hydrogen ion is considered a proton. This is seen in the following reaction, where hydrocyanic acid donates a proton to water, which therefore acts as a base:
HCN + H2O → CN- + H3O+
This reaction is reversible, with the hydronium ion (H3O+) can donate a proton to the CN ion-. Thus, the hydronium ion (H3O+) acts as an acid and the CN- as a base.
CN- + H3O+→ HCN + H2O
*Lewis theory:
This theory was created by the American chemist Gilbert Newton Lewis (1875-1946) and says the following:

Do not stop now... There's more after the advertising ;)
This theory introduces a new concept, is more comprehensive, but does not invalidate the Brönsted-Lowry theory. For every Lewis acid is a Brönsted acid, and therefore every Lewis base is a Brönsted base. This is because a proton receives electrons, meaning a Lewis acid can join a lone pair of electrons in a Lewis base.
For Lewis, an acid-base reaction consists of the formation of a more stable coordinated covalent bond. So when a Lewis base donates a pair of electrons to a Lewis base, they both form a coordinate covalent bond, in which both electrons come from one of the atoms, as in the example bellow:
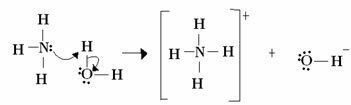
In this case, ammonia acts as the Lewis and Brönsted base, as it donates its two electrons to the proton and is therefore the recipient of the proton. In addition, a covalent bond was formed between hydrogen (the proton) and ammonia.
Water is Lewis acid and Brönsted acid, as it donates the proton and receives electrons, notice how the oxygen in the hydroxide formed from water has an extra pair of electrons.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team.
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Arrhenius, Brönsted-Lowry, and Lewis acid-base theories"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/teorias-acidobase-arrheniusbronstedlowry-lewis.htm. Accessed on June 28, 2021.