French chemist Henri Louis Le Chatelier created one of the best known laws of chemistry that predicts the response of the chemical system in equilibrium when exposed to an alteration.
With the results of his studies, he formulated a generalization for chemical equilibrium that states the following:
"When an external factor acts on a system in equilibrium, it moves, always in the sense of minimizing the action of the applied factor."
When the balance of a chemical system is disturbed, the system acts to minimize this disturbance and restore stability.
Therefore, the system presents:
- an initial state of equilibrium.
- an "unbalanced" state with the change of a factor.
- a new state of equilibrium that opposes change.
Examples of external disturbances that can affect chemical balance are:
| Factor | Disturbance | It is made |
|---|---|---|
| Concentration | Increase | Consume the substance |
| Decrease | the substance is produced | |
| Pressure | Increase | Moves to the smallest volume |
| Decrease | Moves to the highest volume | |
| Temperature | Increase | Absorbs heat and changes the equilibrium constant |
| Decrease | Releases heat and changes the equilibrium constant | |
| Catalyst | Presence | The reaction speeds up |
This principle is of great importance for the chemical industry, as the reactions can be manipulated and make the processes more efficient and economical.
An example of this is the process developed by Fritz Haber, which, using Le Chatelier's principle, economically created a route for the production of ammonia from atmospheric nitrogen.
Next, we'll look at chemical equilibrium according to Chatelier's law and how perturbations can alter it.
know more about:
- Chemical balance
- Ionic Balance
- Acid-base indicators
Concentration effect
When there is a chemical balance, the system is balanced.
The system in equilibrium can suffer a disturbance when:
- We increase the concentration of a component of the reaction.
- We lower the concentration of a component of the reaction.
When we add or remove a substance from the chemical reaction, the system opposes the change, consuming or producing more of that compound so that the balance is re-established.
The concentrations of reactants and products change to adapt to a new equilibrium, but the equilibrium constant remains the same.
Example:
On balance:
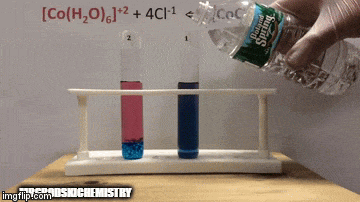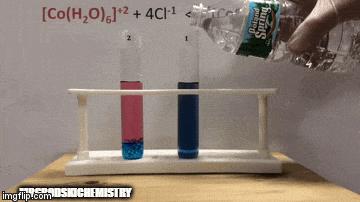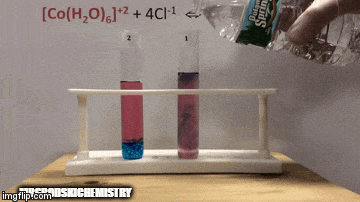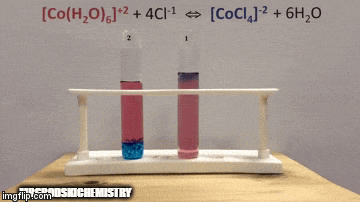
The reaction has a higher concentration of products, because by the blue color of the solution we can see that the complex [CoCl4]-2 predominates.
Water is also a product of the direct reaction and when we increase its concentration in the solution, the system opposes the change, causing the water and the complex to react.
Equilibrium is shifted to the left, reverse reaction direction, and causes the concentration of reactants to increase, changing the color of the solution.
Effect of temperature
The system in equilibrium can suffer a disturbance when:
- There is an increase in system temperature.
- There is a decrease in system temperature.
When adding or removing energy from a chemical system, the system opposes the change, absorbing or releasing energy so that balance is re-established.
When the system varies the temperature, the chemical balance shifts as follows:
By increasing the temperature, the endothermic reaction is favored and the system absorbs heat.
When the temperature is lowered, the exothermic reaction is favored and the system releases heat.
Example:
In chemical balance:
When we place the test tube containing this system in a beaker of hot water, the temperature of the system increases and the equilibrium shifts to form more products.

This is because the direct reaction is endothermic and the system will be re-established by absorbing heat.
Furthermore, temperature variations also alter the equilibrium constants.
pressure effect
The system in equilibrium can suffer a disturbance when:
- There is an increase in total system pressure.
- There is a decrease in total system pressure.
When we increase or decrease the pressure of a chemical system, the system opposes the change, displacing the balance in the sense of smaller or larger volume respectively, but does not change the equilibrium constant.
When the system varies the volume, it minimizes the action of the applied pressure, as follows:
The greater the pressure applied to the system, there will be a contraction of the volume and the equilibrium shifts towards the lower number of moles.
However, if the pressure decreases, the system expands, increasing the volume and the direction of the reaction is shifted to the one with the highest number of moles.
Example:
Our body's cells receive oxygen through chemical balance:
This system is established when the oxygen in the air we breathe comes into contact with the hemoglobin present in the blood, giving rise to oxy-hemoglobin, which carries the oxygen.
When a person climbs a mountain, the higher the altitude reached, the lower the amount and partial pressure of O2 up in the air.
The balance that carries oxygen in the body shifts to the left and reduces the amount of oxy-hemoglobin, compromising the amount of oxygen received by the cells.
The result of this is the appearance of dizziness and fatigue, which can even lead to death.
The body tries to react by producing more hemoglobin. However, this is a slow process, which requires setting at altitude.

Therefore, the people who can climb Mount Everest are the ones who are best suited to extreme altitude.
Catalysts
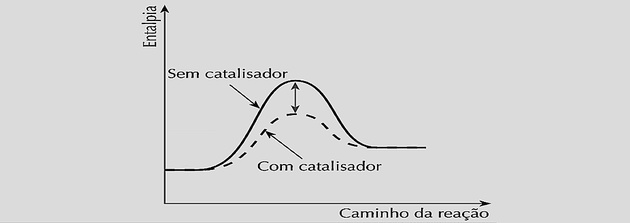
The use of a catalyst interferes with the reaction speed, both in the direct and in the reverse reaction.
For a reaction to occur, it is necessary to reach a minimum energy for the molecules to collide and react effectively.
The catalyst, when inserted into the chemical system, acts by decreasing this activation energy by forming an activated complex and creating a shorter path to reach chemical balance.
By increasing the reaction speeds equally, it reduces the time needed to reach equilibrium, as can be seen in the following graphs:
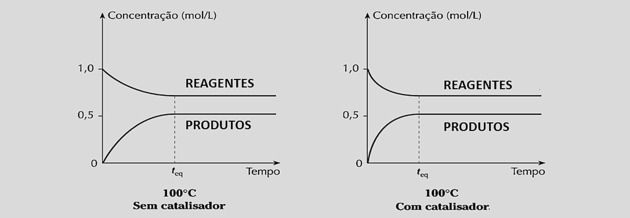
However, the use of catalysts does not change the reaction yield or the equilibrium constant because it does not interfere with the composition of the mixture.
ammonia synthesis
Nitrogen-based compounds are widely used in agricultural fertilizers, explosives, medicines, among others. Due to this fact, millions of tons of nitrogen compounds are produced, such as NH ammonia3, NH ammonium nitrate4AT THE3 and urea H2NCONH2.
Due to the worldwide demand for nitrogen compounds, mainly for agricultural activities, Chile's NaNO saltpeter3, the main source of nitrogenous compounds, was the most used until the beginning of the 20th century, but natural saltpeter would not be able to supply the current demand.
It is interesting to note that atmospheric air is a mixture of gases, composed of more than 70% nitrogen N2. However, due to the stability of the triple bond it becomes a very difficult process to break this bond to form new compounds.
The solution to this problem was proposed by the German chemist Fritz Haber. The synthesis of ammonia proposed by Haber brings the following chemical balance:
To be industrially implemented, this process was perfected by Carl Bosch and is the most used to date for capturing nitrogen from the air with a focus on obtaining nitrogenous compounds.
Using Le Chatelier's principle, chemical balance can be increased when:
Add H2 and causes the system to oppose the change and react to lower the concentration of that reactant.
Thus, H2 and no2 they are consumed simultaneously to produce more product and create a new equilibrium state.
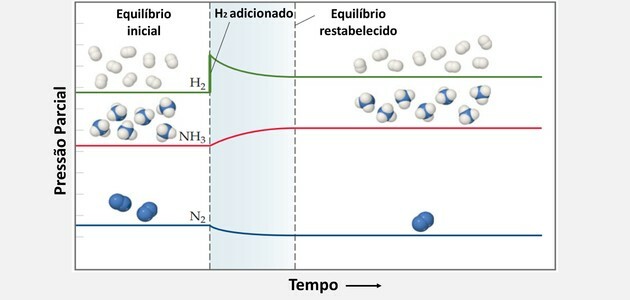
Likewise, by adding more nitrogen, the balance shifts to the right.
Industrially, the balance is shifted by the continuous removal of NH3 of the system through selective liquefaction, increasing the reaction yield, as the balance to be re-established tends to form more product.
Haber-Bosch synthesis is one of the most important applications of chemical equilibrium studies.
Due to the relevance of this synthesis, Haber received the Nobel Prize in Chemistry in 1918 and Bosch was awarded the Prize in 1931.
Balance shift exercises
Now that you know how to interpret the changes that can occur in chemical balance, use these vestibular questions to test your knowledge.
1. (UFPE) The most suitable antacids should be those that do not reduce the acidity in the stomach too much. When the reduction in acidity is too great, the stomach secretes excess acid. This effect is known as the “acid rematch”. Which of the items below could be associated with this effect?
a) The law of energy conservation.
b) The Pauli exclusion principle.
c) The principle of Le Chatelier.
d) The first principle of Thermodynamics.
e) Heisenberg's uncertainty principle.
Correct alternative: c) Le Chatelier's principle.
Antacids are weak bases that work by increasing the pH of the stomach and, consequently, decreasing the acidity.
The decrease in acidity occurs by neutralizing the hydrochloric acid present in the stomach. However, by reducing the acidity too much, it can create an imbalance in the body, as the stomach works in an acidic environment.
As stated by Le Chatelier's principle, when an equilibrium system is exposed to a disturbance, there will be opposition to this change so that the equilibrium is re-established.
In this way, the body will produce more hydrochloric acid producing the “acid rematch” effect.
The other principles presented in the alternatives deal with:
a) The law of energy conservation: in a series of transformations, the total energy of the system is conserved.
b) The Pauli exclusion principle: in an atom, two electrons cannot have the same set of quantum numbers.
d) The first principle of Thermodynamics: the variation of the system's internal energy is the difference between heat exchanged and work performed.
e) Heisenberg's uncertainty principle: it is not possible to determine the speed and position of an electron at any given instant.
Regarding the system in equilibrium, it can be correctly stated that:
a) the presence of a catalyst affects the composition of the mixture.
b) the presence of a catalyst affects the equilibrium constant.
c) the increase in pressure decreases the amount of CH4(g).
d) the increase in temperature affects the equilibrium constant.
e) the increase in temperature decreases the amount of CO(g) .
Correct alternative: d) the increase in temperature affects the equilibrium constant.
When raising the temperature, the direct reaction, which is endothermic, will be affected, because to re-establish the balance the system will absorb energy and shift the balance to the right.
Shifting the balance in the direct direction, the amount of formed products is increased.
The equilibrium constant is directly proportional to the concentration of products: the greater the quantity of products, the greater the value of the constant.
We can observe then, that the increase in temperature increases the amount of CO and H2.
The increase in pressure shifts the equilibrium to the reverse reaction, as the equilibrium shifts towards the lowest number of moles. With that, the amount of CH4 and H2The is augmented.
The use of catalyst does not interfere with the equilibrium constant and composition of the mixture. It will only act to make the balance more quickly achieved.
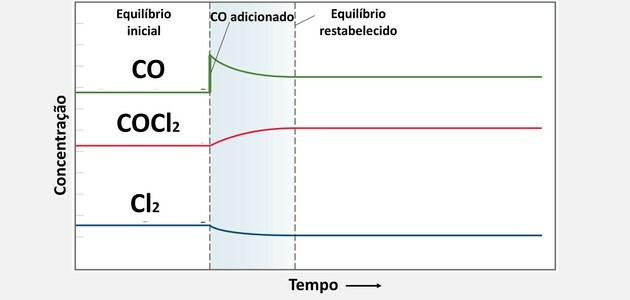
3. (UFC) In the study of the action of COCl poison gas2, used as a chemical weapon, the decomposition process is observed according to the reaction:
Starting from an equilibrium situation, 0.10 mol of CO was added and the system, after some time, reached a new equilibrium situation. Choose the option that indicates how the new equilibrium concentrations are related to the old ones.
| [COCl2] | [CO] | [Cl2] | |
| The) | new > old | new > old | new |
| B) | new > old | new > old | new > old |
| ç) | new | new > old | new |
| d) | new > old | new | new |
| and) | same | same | same |
Correct alternative:
| [COCl2] | [CO] | [Cl2] | |
| The) |
When a new substance is added, the system consumes that substance to restore balance, as its concentration has increased.
This consumption occurs by making the substance react with the other compound, thus creating more product.
Therefore, when we increase the concentration of CO, there will be consumption, but not to the point of becoming lower than the concentration in the initial state, as its consumption will occur together with another component.
Already the concentration of Cl2 becomes smaller than the initial one, as it had to react with the amount of CO added.
From the junction of the two substances, the concentration of COCl was increased2, as it is the product formed.
These changes in chemical balance can be seen in the graph below:
4. (UFV) The experimental study of a chemical reaction in equilibrium demonstrated that the increase in temperature favored the formation of products, while the increase in pressure favored the formation of reagents. Based on this information, and knowing that A, B, C and D are gases, mark the alternative that represents the studied equation:
| The) | ||
| B) | ||
| ç) | ||
| d) | ||
| and) |
Correct alternative:
| The) |
As the temperature increases, the system absorbs heat to restore balance and, with this, favors the endothermic reaction, whose ∆H is positive.
The alternatives that correspond to favoring the formation of products by increasing the temperature are: a, b and d.
However, when the pressure increases, the equilibrium shifts towards the smallest volume, that is, the one with the smallest number of moles.
For the reaction to be moved towards the reactants, it is necessary that this direction of the reaction has a smaller number of moles in relation to the products.
This is only observed in the first alternative.
5. (UEMG) The following equations represent systems in equilibrium. What is the only system that does not shift by pressure change?
a) OS2(g) + 1/2 O2(g) ⇔ SO3(g)
b) CO2(g) + H2(g) ⇔ CO(g) + H2O(g)
c) No2(g) + 3 H2(g) ⇔ 2 NH3(g)
d) 2 CO2(g) ⇔ 2 CO(g) + O2(g)
Correct alternative: b) CO2(g) + H2(g) ⇔ CO(g) + H2O(g)
When a system changes total pressure, balance is re-established with the change in volume.
If pressure increases, volume decreases, shifting equilibrium to the smallest number of moles.
On the other hand, when the pressure decreases, the volume increases, shifting the balance towards a greater number of moles.
But when there are the same number of moles of reacting substances and products formed, there is no way to shift the equilibrium, as the volume does not change.
We know the number of moles by the stoichiometric coefficients next to each substance.
We can see this in the alternative equation
b) CO2(g) + H2(g) ⇔ CO(g) + H2O(g)
where 1 mole of CO2 reacts with 1 mole of H2 to form 1 mol of CO and 1 mol of H2O.
In both directions of the reaction there are 2 moles, so changes in pressure would not change the volume.
Check out more questions about chemical equilibrium shift, with commented resolution, in this list we have prepared: chemical balance exercises.
Who was Le Chatelier?