THE ozonolysis it is a type of efficient reaction for the oxidation of an unsaturated compound with the breaking of its double bond. Is it over there occurs between a alkene (alkene) and an ozone (O3), as an oxidizing agent. Then hydrolysis occurs when reacting with the water (H2O) in the presence of zinc powder or zinc filings (Zn) as catalysts, forming the main products: aldehydes and/or ketones, in addition to hydrogen peroxide (H2O2).
In a simplified way we can say that ozonolysis is a reaction with ozone followed by hydrolysis. Its chemical equation is written as follows, considering a generic alkene:
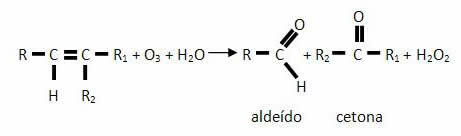
It is precisely because of the formation of hydrogen peroxide that zinc powder is introduced, as it destroys water Oxygen formed, preventing the oxygen that can be produced by its decomposition from oxidizing the aldehyde to acid carboxylic acid.
This reaction first forms a stable intermediate compound called a ozone or ozonide, or yet ozone. This is a product of the bonding of oxygen atoms in ozone with the carbons that form the double bond of alkene. Ozone is an unstable and explosive compound.
Do not stop now... There's more after the advertising ;)
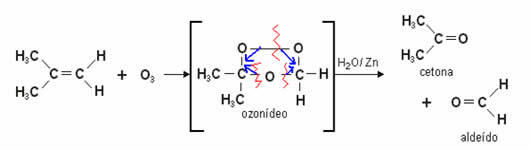
We observe that the carbon-carbon double bond (C=C) is broken and they start to participate of the double bond with oxygen (C=O) in the newly formed substances which may be aldehydes or ketones.
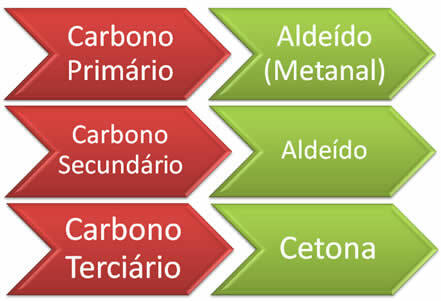
To know whether the formation of aldehydes or ketones will occur depends on the initial alkene molecule, that is, depends on the location of the double in the alkene and whether the carbon attached to the double bond is primary, secondary or tertiary. Furthermore, the unknown structure of the alkene can also be determined by the products formed. So, we have the following rule:
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Ozonolysis of alkenes"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/ozonolise-alcenos.htm. Accessed on June 28, 2021.