Inorganic functions are groups of inorganic compounds that have similar characteristics.
A fundamental classification in relation to chemical compounds is: organic compounds are those that contain carbon atoms, while organic compounds contain carbon atoms. inorganic compounds they are formed by the other chemical elements.
There are exceptions such as CO, CO2 and on2CO3, which despite having carbon in the structural formula, have characteristics of inorganic substances.
The four main inorganic functions are: acids, bases, salts and oxides.
These 4 main functions were defined by Arrhenius, a chemist who identified ions in acids, bases and salts.
Acids
Acids they are covalent compounds, that is, they share electrons in their bonds. They have the ability to ionize in water and form charges, releasing H+ as the only cation.
Classification of acids
Acids can be classified according to the amount of hydrogen that is released into an aqueous solution and ionize, reacting with water to form the hydronium ion.
| Number of ionizable hydrogens |
|---|
|
Monoacids: they have only one ionizable hydrogen. Examples: HNO3, HCl and HCN |
|
diacids: have two ionizable hydrogens. Examples: H2ONLY4, H2S and H2MnO4 |
|
Triacids: have three ionizable hydrogens. Examples: H3DUST4 and H3BO3 |
|
tetracids: have four ionizable hydrogens. Examples: H4P7O7 |
The strength of an acid is measured by the degree of ionization. The higher the value of stronger is the acid because:
| degree of ionization |
|---|
|
strong: have a degree of ionization greater than 50%. |
|
moderate: have a degree of ionization between 5% and 50%. |
|
weak: have a degree of ionization below 5%. |
Acids may or may not contain the element oxygen in their structure, thus:
| presence of oxygen |
|---|
|
Hidracids: do not have oxygen atoms. Examples: HCl, HBr and HCN. |
|
oxyacids: The element oxygen is present in the acid structure. Examples: HClO, H2CO3 and HNO3. |
Acid nomenclature
The general formula of an acid can be described as HxTHE, where A represents the anion that makes up the acid and the nomenclature generated can be:
| anion termination | Acid Termination |
|---|---|
|
etho Example: Chloride (Cl-) |
hydric Example: hydrochloric acid (HCl) |
|
act Example: chlorate |
ich Example: chloric acid (HClO3) |
|
very Example: nitrite |
bone Example: nitrous acid (HNO2) |
Characteristics of acids
The main characteristics of acids are:
- They taste sour.
- They carry electrical current, as they are electrolytic solutions.
- They form hydrogen gas when they react with metals such as magnesium and zinc.
- Form carbon dioxide when reacting with calcium carbonate.
- They change the acid-base indicators to a specific color (blue litmus paper turns red).
Main acids
Examples: hydrochloric acid (HCl), sulfuric acid (H2ONLY4), acetic acid (CH3COOH), carbonic acid (H2CO3) and nitric acid (HNO3).
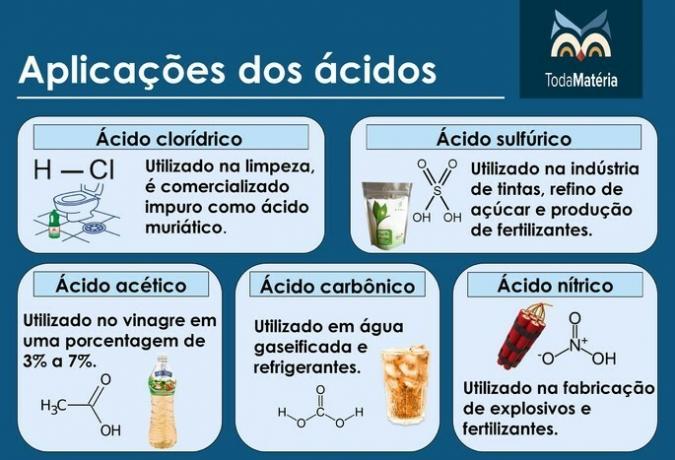
Although acetic acid is an acid from Organic Chemistry, it is important to know its structure because of its importance.
Bases
Bases are ionic compounds formed by cations, mostly metals, which dissociate in water releasing the hydroxide anion (OH-).
Base classification
Bases can be classified according to the number of hydroxyls released into solution.
| Number of hydroxyls |
|---|
|
Monobases: they have only one hydroxyl. Examples: NaOH, KOH and NH4oh |
|
Dibases: have two hydroxyls. Examples: Ca(OH)2, Fe(OH)2 and Mg(OH)2 |
|
Tribases: have three hydroxyls. Examples: Al(OH)3 and Fe(OH)3 |
|
tetrabases: have four hydroxyls. Examples: Sn(OH)4 and Pb(OH)4 |
Bases are generally ionic substances and the strength of a base is measured by the degree of dissociation.
The higher the value of stronger is the base because:
| dissociation degree |
|---|
|
strong: they have a degree of dissociation practically 100%. Examples:
|
|
weak: have a degree of dissociation less than 5%. Example: NH4OH and Zn(OH)2. |
| Solubility in water |
|---|
|
Soluble: alkali metal and ammonium bases. Examples: Ca(OH)2, Ba(OH)2 and NH4Oh. |
|
Slightly soluble: alkaline earth metal bases. Examples: Ca(OH)2 and Ba(OH)2. |
|
practically insoluble: other bases. Examples: AgOH and Al(OH)3. |
Base nomenclature
The general formula of a base can be described as , where B represents the positive radical that makes up the base and y is the charge that determines the number of hydroxyls.
The nomenclature for bases with fixed load is given by:
| Bases with fixed load | ||
|---|---|---|
alkali metals |
lithium hydroxide |
LiOH |
| Alkaline Earth Metals | magnesium hydroxide |
Mg(OH)2 |
Silver |
silver hydroxide |
AgOH |
| Zinc | zinc hydroxide | Zn(OH)2 |
| Aluminum | aluminum hydroxide | Al(OH)3 |
When the base has a variable load, the nomenclature can be in two ways:
| Bases with variable load | |||
|---|---|---|---|
| Copper | Ass+ | copper hydroxide I | CuOH |
| cuprous hydroxide | |||
| Ass2+ | copper hydroxide II | Cu(OH)2 | |
| cupric hydroxide | |||
| Iron | Faith2+ | Iron hydroxide II | Fe(OH)2 |
| ferrous hydroxide | |||
| Faith3+ | Iron hydroxide III | Fe(OH)3 | |
| ferric hydroxide |
Characteristics of the bases
- Most bases are insoluble in water.
- Conduct electric current in aqueous solution.
- They are slippery.
- They react with acid to form salt and water as products.
- They change the acid-base indicators to a specific color (red litmus paper turns blue).
Main bases
Bases are widely used in cleaning products and also in chemical industry processes.
Examples: sodium hydroxide (NaOH), magnesium hydroxide (Mg (OH)2), ammonium hydroxide (NH4OH), aluminum hydroxide (Al(OH)3) and calcium hydroxide (Ca(OH)2).

salts
salts are ionic compounds that have at least one cation other than H+ and an anion other than OH-.
A salt can be obtained in a neutralization reaction, which is the reaction between an acid and a base.
The reaction of hydrochloric acid with sodium hydroxide produces sodium chloride and water.
The salt formed is composed of the acid anion (Cl-) and by the base cation (Na+).
Classification of salts
Below, we have the main families of salts that can be classified according to water solubility and pH change of the solution as follows:
| Water solubility of the most common salts | |||
|---|---|---|---|
| Soluble | Nitrates | Exceptions: Silver acetate. |
|
| Chlorates | |||
Acetates |
|||
| Chlorides | Exceptions: |
||
| Bromides | |||
| Iodides | |||
| Sulphates |
Exceptions: |
||
| Insoluble | Sulfides |
Exceptions: alkaline earth and ammonium. |
|
| Carbonates | Exceptions: Those of alkali metals and ammonium. |
||
| Phosphates |
| pH | |
|---|---|
| neutral salts |
When dissolved in water they do not change the pH. Example: NaCl. |
| acid salts |
When they are dissolved in water they make the solution pH less than 7. Example: NH4Cl. |
| basic salts |
When they are dissolved in water they make the solution pH greater than 7. Example: CH3COONa. |
In addition to the salt families we saw earlier, there are other types of salts, as shown in the table below.
| Other types of salts | |
|---|---|
| hydrogen salts | Example: NaHCO3 |
| Hydroxy-salts | Example: Al(OH)2Cl |
| double salts | Example: KNaSO4 |
| hydrated salts | Example: CuSO4. 5 hours2O |
| complex salts | Example: [Cu (NH3)4]ONLY4 |
Nomenclature of salts
In general, the nomenclature of a salt follows the following order:
| anion name | cation name | name of salt |
|---|---|---|
|
Cl- Chloride |
Faith3+ Iron III |
FeCl3 Iron Chloride III |
|
Sulfate |
At+ Sodium |
At2ONLY4 Sodium Sulfate |
|
Nitrite |
K+ Potassium |
KNO2 Potassium nitrite |
|
br- Bromide |
Here2+ Calcium |
CaBr2 calcium bromide |
Characteristics of salts
- They are ionic compounds.
- They are solid and crystalline.
- Suffer from boiling at high temperatures.
- Conduct electric current in solution.
- They taste salty.
Main salts
Examples: potassium nitrate (KNO3), sodium hypochlorite (NaClO), sodium fluoride (NaF), sodium carbonate (Na2CO3) and calcium sulfate (CaSO4).

Oxides
Oxides are binary compounds (ionic or molecular) that have two elements. They have oxygen in their composition, which is their most electronegative element.
The general formula for an oxide is , where C is the cation and its charge y becomes an index in the oxide forming the compound:
Classification of oxides
| According to chemical bonds | |
|---|---|
| Ionic |
Combination of oxygen with metals. Example: ZnO. |
| Molecular |
Combination of oxygen with non-metallic elements. Example: OS2. |
| According to properties | |
|---|---|
| Basics |
In aqueous solution they change the pH to greater than 7. Example: I read2O (and other alkali and alkaline earth metals). |
| Acids |
In aqueous solution they react with water and form acids. Examples: CO2, ONLY3 and NO2. |
| Neutrals |
Some oxides that do not react with water. Example: CO. |
| Peroxides |
In aqueous solution they react with water or diluted acids and form hydrogen peroxide H2O2. Example: Na2O2. |
| Amphoters |
They can behave like acids or bases. Example: ZnO. |
Nomenclature of oxides
In general, the nomenclature of an oxide follows the following order:
| Name according to type of oxide | |
|---|---|
| ionic oxides |
Examples of fixed charge oxides: CaO - calcium oxide Al2O3 - aluminum oxide |
|
Examples of oxides with variable charge: FeO - Iron Oxide II Faith2O3 - Iron oxide III | |
| molecular oxides |
Examples: CO - carbon monoxide N2O5 - Dinitrogen pentoxide |
Oxide characteristics
- They are binary substances.
- They are formed by the binding of oxygen with other elements, except fluorine.
- Metal oxides, when reacting with acids, form salt and water.
- Non-metallic oxides, when reacting with bases, form salt and water.
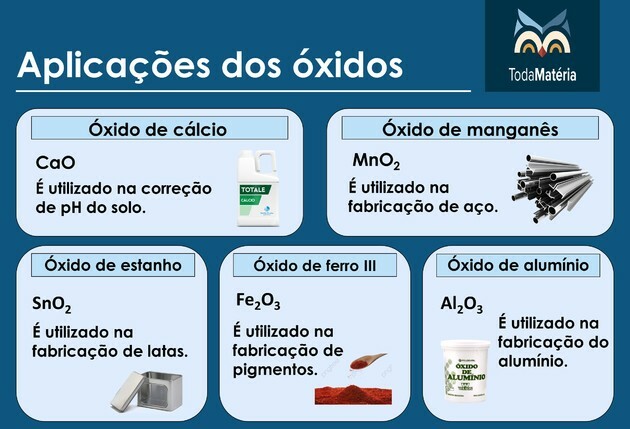
Main oxides
Examples: calcium oxide (CaO), manganese oxide (MnO2), tin oxide (SnO2), iron oxide III (Fe2O3) and aluminum oxide (Al2CO3).
Entrance Exam Exercises
1. (UEMA/2015) NO2and the OS2 are gases that cause atmospheric pollution that, among the damage caused, result in the formation of of acid rain when these gases react with water particles present in clouds, producing HNO3 and H2ONLY4.
These compounds, when carried by atmospheric precipitation, generate disturbances, such as contamination of drinking water, corrosion of vehicles, historical monuments, etc.
The inorganic compounds mentioned in the text correspond, respectively, to the functions:
a) salts and oxides
b) bases and salts
c) acids and bases
d) bases and oxides
e) oxides and acids
Correct alternative: e) oxides and acids.
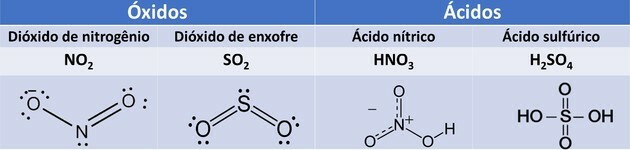
Oxides are compounds formed by oxygen and other elements, except fluorine.
Acids, when they come into contact with water, undergo ionization and produce the hydronium ion. For the acids in question, we have the following reactions:
the HNO3 it is monoacid because it has only one ionizable hydrogen. the H2ONLY4 it is a diacid because it has two ionizable hydrogens.
The other inorganic functions present in the questions correspond to:
Bases: hydroxyl ions (OH-) ionically bonded with metal cations.
Salts: product of the neutralization reaction between an acid and a base.
Learn more aboutchemical functions.
2. (UNEMAT/2012) We make use of various chemical products in our daily lives, such as magnesium milk, vinegar, limestone and caustic soda.
It is correct to state that these substances mentioned belong, respectively, to the chemical functions:
a) acid, base, salt and base
b) base, salt, acid and base
c) base, acid, salt and base
d) acid, base, base and salt
e) salt, acid, salt and base
Correct alternative: c) base, acid, salt and base.
Magnesium milk, limestone and caustic soda are examples of compounds that contain inorganic functions in their structures.
Vinegar is an organic compound formed by a weak carboxylic acid.
In the table below we can observe the structures of each one of them and the chemical functions that characterize them.
| Product | Magnesium milk | Vinegar | Limestone | Caustic soda |
|---|---|---|---|---|
| Chemical compost | magnesium hydroxide | Acetic Acid | Calcium carbonate | Sodium hydroxide |
| Formula | ||||
| chemical function | Base | carboxylic acid | salt | Base |
Magnesium milk is a suspension of magnesium hydroxide used to treat stomach acid, as it reacts with hydrochloric acid from gastric juice.
Vinegar is a widely used condiment mainly in food preparation due to its aroma and flavor.
Limestone is a sedimentary rock, whose main ore is calcite, which contains large amounts of calcium carbonate.
Caustic soda is the trade name for sodium hydroxide, a strong base used in many industrial processes and household use to unclog pipes due to an accumulation of oils and grease.
3. (UDESC/2008) Regarding hydrochloric acid, it can be said that:
a) when in aqueous solution, it allows the passage of electric current
b) is a diacid
c) is a weak acid
d) has a low degree of ionization
e) is an ionic substance
Correct alternative: a) when in aqueous solution, it allows the passage of electric current.
Hydrochloric acid is a monoacid as it has only one ionizable hydrogen.
It is a molecular compound, with a high degree of ionization and therefore it is a strong acid, which, when entering into contract with water, breaks its molecule into ions as follows:
As Arrhenius observed in his experiments, the positive ions formed in ionization move towards the negative pole, while the negative ions move towards the positive pole.
In this way, electrical current flows into the solution.
For more issues with commented resolution, see also: exercises on inorganic functions.



