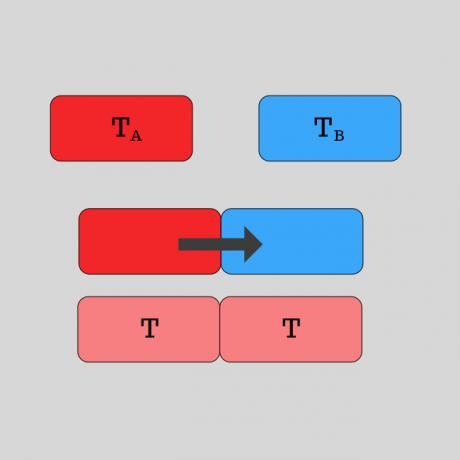
Thermal equilibrium happens when two bodies - initially at different temperatures - they come into contact and after a heat transfer process, reach the same temperature.
Heat, the name given to thermal energy in physics, is always transferred from the hottest body to the coldest body, and when the bodies reach the same temperature, the heat flow between them is suspended.
If there is no heat exchange with the environment, the amount of heat lost by one body is equal to the amount of heat absorbed by the other.

Image: illustration of two bodies in thermal equilibrium after contact.
The same can happen with a body whose parts are at different temperatures. When the whole body reaches the same temperature, it will be in thermal equilibrium.
Heat
In everyday language, the word heat is related to the sensation in our body when the temperature is high. In physics, the meaning of heat is different. The heat, in this case, is the thermal energy in motion.
Bodies are composed of particles and heat is determined by the degree of agitation of these particles. That is, the greater the agitation of its molecules, the greater the thermal energy, or heat.
Heat is measured in joule (J) or calorie (lime). Calorie is the amount of energy needed to raise by 1°C, 1g of water. The relationship between these units of measure is: 1 cal = 4.186 j.
How does thermal equilibrium happen?
When two bodies are at different temperatures, their molecules are at different degrees of agitation. The molecules of the higher temperature body are more agitated and the lower temperature less agitated.
When they come into contact, the body with the highest temperature will transfer thermal energy to the other, until both reach the same temperature, which is called thermal equilibrium. To make it easier, let's think of an example:
To make gelatine you need to mix the powder in boiling water and then put the same amount of water at room temperature. Water that is at 100°C will come into contact with water at room temperature and will transfer heat, until all the liquid in the container has the same temperature.
If there was no heat exchange with the environment, the final temperature would be exactly the average of the two temperatures. See the example:
Water at 100°C + Water at 25°C = 125°C
This value divided by 2 would equal 62.5°. However, as the mixture loses energy to the environment, this temperature, in practice, will be a little lower.
the thermometer
When we need to measure body temperature, we use a thermometer. This device tells us the temperature with thermodynamic balance between the body and the thermometer.
When the thermometer reaches the same temperature as our body, it stops transferring heat and, in some cases, it beeps to let you know that the flow has stopped. And so we know if we have a fever or not.
know more about specific heat and temperature.
Ways of heat transmission
Energy in the form of heat can be transmitted from one body to another in three ways: conduction, convection and radiation.
Driving
Conduction occurs when energy is transferred from molecule to molecule. Molecules with greater agitation come into contact with molecules with less agitation and increase their speed of movement.
If you hold a metal spoon at one end and touch the other end to the fire, you can in a few moments burn your hand. This is because the molecules stirred by fire cause the other molecules to stir, bringing thermal energy to all the cutlery - increasing its temperature.
Convection
Convection occurs with gaseous or liquid substances, which transfer heat through currents. circular shapes that form due to the difference in density of the hot and cold parts of a substance.
This is the case of a pot heating water. The part of the water that is closest to the fire will heat up first, as the density of hot water is lower, it will rise in the pan and the coldest part of the water will go down, thus forming a circular current of convection.
Irradiation
In irradiation it is not necessary for there to be contact between bodies for the energy to propagate, as occurs in conduction or convection. In this case, thermal energy is propagated from one body to another by electromagnetic waves. The body that receives heat is called the receiver and the one that emits is called the emitter.
This is how the sun warms planet Earth. As we know, the sun is very far from Earth, that is, there is no contact. The heat that reaches Earth and guarantees life on the planet is transmitted by radiation, through electromagnetic waves.
See also the meaning of heat and Thermal energy.