Chlorofluorocarbons (CFCs) are chemical compounds formed by three elements: carbon (Ç), fluorine (Faith chlorine (Cl).
These compounds are part of the organic halides family and are synthesized from hydrocarbons by replacing hydrogen atoms with halogens.
There are several compounds of CFCs and the most common representatives are:
- CFC 11 - trichlorofluoromethane (CCl3F)
- CFC 12 - dichlorodifluoromethane (CCl2F2)
- CFC 113 - trichlorotrifluoroethane (C2Cl3F3)
CFCs became popularly known because of Freon, a set of refrigerant gases based on chlorofluorocarbons.
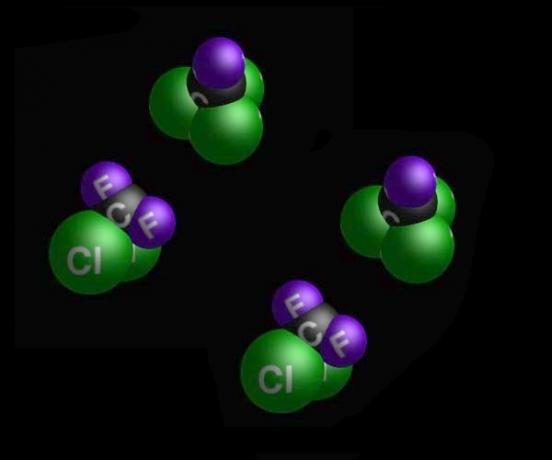 Trichlorofluoromethane (CCl3F) and dichlorodifluoromethane (CCl2F2).
Trichlorofluoromethane (CCl3F) and dichlorodifluoromethane (CCl2F2).
Characteristics of chlorofluorocarbons
The main characteristics of these compounds are as follows:
- They are volatile.
- They are good solvents.
- They have low boiling points.
Halogens attached to carbon atoms make the compound more volatile and less reactive. These were precisely the characteristics sought by the industry when they developed CFCs in 1930, as a safer alternative to replacing ammonia and sulfur dioxide.
The most common applications of CFCs were for refrigeration purposes, foam expander, can propellant. spray and fire systems.
Chlorofluorocarbon and the ozone layer
Although there is no significant amount of chlorine in the atmosphere, CFCs have increased the presence of this chemical in the ozone layer. The layer is around the Earth, at an altitude of approximately 15 to 20 km and is formed by ozone gas (O3).
Chlorine atoms and free radicals are produced at this altitude by the decomposition of CFCs generated by ultraviolet radiation, causing enormous damage to the ozone layer.
The preservation of this layer is essential for the conservation of health and survival of all living beings, as it is responsible for being a protective barrier against the ultraviolet rays emanating from the sun.
If the ozone layer did not exist, life on Earth would be extinct, as ultraviolet rays with direct incidence would be fatal to living beings.
Between the 1940s and 1970s chlorofluorocarbons were widely used in various products, mainly in versions of products presented in spray, in air conditioners and in gases from refrigeration systems.
However, some time later, starting in the 1970s, it was discovered that the emission of chlorofluorocarbons into the atmosphere was one of the main factors responsible for the increase in the hole in the ozone layer.
Hole in the ozone layer
In recent years, the ozone layer has suffered great damage as a result of some processes linked to the growth and modernization of society.
Some substances used by man are the causes of the progressive destruction that the layer has been suffering. The main responsible for this process and for the increase of the hole are the emissions of chlorofluorocarbons (CFCs).
The bigger the hole in the ozone layer, the greater the damage suffered by all life on Earth. This happens because the thinning of the layer increases the incidence of ultraviolet rays that reach living beings.
 The darkest part of the image indicates the proportion of the hole in the ozone layer.
The darkest part of the image indicates the proportion of the hole in the ozone layer.
One of the most common health problems related to the incidence of ultraviolet rays on Earth is the skin cancer. The incidence of the disease has grown worldwide and, according to the World Health Organization (WHO), the growth of cases must receive urgent attention so that measures can be taken. prevention.
The number of new cases of skin cancer has already surpassed the mark of 15 million per year and, if the problem is not contained, it should increase even more in the coming periods.
The hole in the ozone layer and the greenhouse effect
The greenhouse effect, contrary to what many people might think, is a naturally occurring phenomenon that is fundamental for maintaining the Earth's temperature.
The effect guarantees the retention of part of the heat emitted by the sun's rays, which keeps the planet's atmosphere warm, guaranteeing the survival of living beings.
If the greenhouse effect did not exist, when the sun's rays reached the Earth's surface, they would return to extraterrestrial space and heat would be lost.
However, for the greenhouse effect to fulfill its function and maintain healthy life on Earth, the temperature must be kept balanced.
 Incidence of the sun's rays on the Earth's surface.
Incidence of the sun's rays on the Earth's surface.
Due to the emission of gases harmful to the greenhouse effect and the increase in the hole in the ozone layer, a part of the solar radiation, which was supposed to be reflected back to space, is being retained on the surface of the planet.
This retention causes temperatures to rise, increasing the planet's temperature in excess. This phenomenon is known as global warming.
Excessive heating causes various types of imbalances on the planet, as it alters the frequency of rains, increases heat waves, harms living beings and alters water levels in oceans.
Control of CFCs in the atmosphere
As soon as the first notices of damage caused by CFCs were published, replacement searches began of these compounds by other products with less destructive potential, in addition to the search for alternatives that were not halocarbons.
One of the milestones to minimize this environmental problem occurred in 1990, with the signing of the Montreal Protocol. The agreement was signed by 93 countries to end the production of chemical substances that deplete the ozone layer.
In 1992, the list of countries that adhered to the target grew to 140, which demonstrates the intention to control emissions.
Brazil was one of the countries that committed to continuously reducing CFCs and taking other measures to alleviate the problems caused by production and use.
One of the alternatives to replace harmful gases was the use of HCFCs, as they present a lower risk, since they decompose in lower atmospheres.
However, chlorine accumulation continued to be observed and it was concluded that they were also degrading the ozone layer.
Refrigeration systems had as alternatives fluids composed of hydrocarbons, such as propane and isobutane used in air conditioning systems in Australia, the United States and many others countries.
See also the meanings of Ozone, Ozone layer, Greenhouse effect and Global warming.
