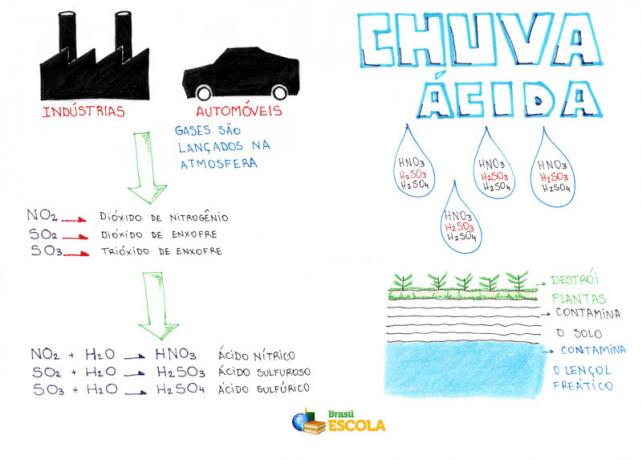
Acid rain is produced by oxides: Sulfur dioxide (SO2) and nitrogen (NO2), both derived from burning coal, fossil fuels and industrial pollutants.
ONLY2 and NO2 they then become components of our atmosphere. And so the problem arises: these gases, combined with the hydrogen present in the atmosphere (water vapour), give rise to rain loaded with sulfuric acid and nitric acid. As can be seen, the presence of these acids is what makes the rain acidified.
When acid rain falls to the surface, it causes a great environmental impact, alters the chemical composition of soil and water, affects food chains, and destroys forests and crops. What's more, they cause damage not only in the countryside, but also in cities: they corrode metallic structures, historical monuments (statues) and buildings.
Let's know a little more about the action of these acids:
→ sulfuric acid (H2ONLY4)
This acid can destroy paper, cotton fabrics, wood, sugar and other materials due to its energetic action (dehydrating). Imagine the damage it causes when present in the rain?
Sulfuric acid has a corrosive action on the tissues of living organisms. Rains formed in an environment polluted with sulfur dioxide contain H2ONLY4, which causes a great destructive impact, due to its property of corroding plants, metals and even stones, such as marble for example.
Do not stop now... There's more after the advertising ;)
→ Nitric acid (HNO3)
Acid rain produced in environments polluted with nitrogen oxide (NO) contains in its composition the acid HNO3.
Nitric acid is toxic and, like sulfuric acid, it is corrosive and causes a lot of damage to nature.
Mind Map: Acid Rain
*To download the mind map in PDF, Click here!
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Chemistry of Acid Rain"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/quimica-chuva-acida.htm. Accessed on June 27, 2021.