THE thermal capacity determines the amount of heat that a body needs to receive to change its temperature in one unit. Each body behaves differently when receiving a certain amount of heat, and an example where we can easily see this occurs on the beach. Sand and seawater are subject to the same heat source, the sun, but sand is much hotter than water. This is because sand and water have different thermal capacities.
Therefore, the thermal capacity is a quantity that depends on the amount of heat received and the variation of temperature suffered by a body. It can be defined as follows:
“The thermal capacity (C) is the ratio between the amount of heat (Q) received by a body and the temperature variation (ΔT) suffered by it.”
Mathematically, this relationship is given by the expression:
C = Q
ΔT
The unit of measurement for thermal capacity in the International System is calories per degree Celsius (cal/ºC). An example to better understand the interpretation of this quantity is the following situation:
If a body receives 1000 cal and increases its temperature by 20°C, its heat capacity is?
C = Q = 1.000 = 50 cal/°C
ΔT 20
In other words, for every 50 calories that the body receives, its temperature varies by 1°C.
Do not stop now... There's more after the advertising ;)
Thermal capacity is a property of bodies that depends only on their mass, so two bodies made of the same material may experience different temperature variations when receiving the same amount of heat if their masses are many different. For example, if we want to heat two sheets of metal, one with 5 kg and one with 10 kg, it will be necessary to provide more heat to the 10 kg sheet, as its mass is greater.
Mind Map: Thermal Capacity
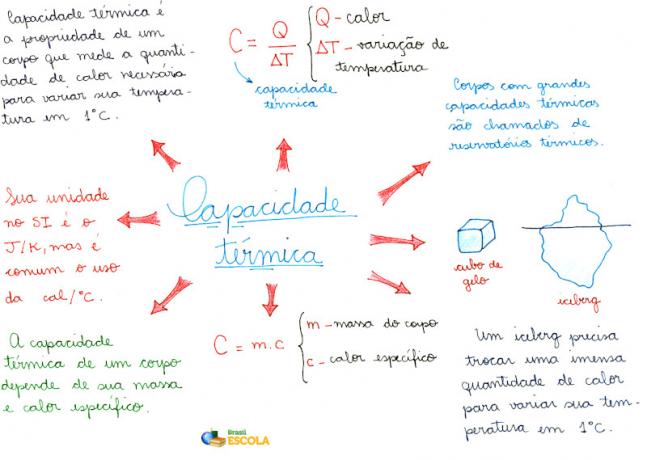
* To download the mind map in PDF, Click here!
We can conclude, therefore, that the thermal capacity is proportional to the mass of the bodies. This proportionality is defined by a quantity called specific heat (c), which is determined by the constant ratio between the heat capacity and the mass of a substance, being expressed mathematically by the equation:
c = Ç
m
The unit of measurement for the specific heat is cal/g.ºC. This quantity defines the amount of heat that must be supplied or removed from each 1 gram of a material to vary its temperature by 1°C.
The thermal capacity and specific heat of the materials can be determined using a calorimeter, a device with thermal insulation used to study the heat exchange between bodies of different temperatures.
Mariane Mendes
Graduated in Physics
*Mental Map by Me. Rafael Helerbrock
Would you like to reference this text in a school or academic work? Look:
TEIXEIRA, Mariane Mendes. "Thermal capacity"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/capacidade-termica.htm. Accessed on June 27, 2021.