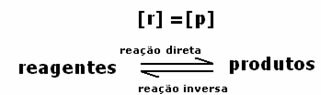
The number of universities that use Enem as a resource for the admission of students has grown every year, which increases the relevance of this exam.
To help you in this process, we've selected topics on what to study in general chemistry for Enem. Come on?
1- Physical states of matter and their transformations
Matter is everything that occupies space and has mass, and can be found in some physical states and undergo some transformations. The main topics related to this subject are:
physical states of matter
Graphs of Physical State Changes
2- Properties of matter
Matter has several characteristics that are decisive in understanding various contents of Chemistry, such as separation of mixtures. The main topics referring to the characteristics of the matter are:
properties of matter
General and specific properties
Density
Intensive and extensive properties
Melting and boiling point
Solubility
Physical and chemical phenomena
3- Characteristics of substances and mixtures
Knowing how the atoms of elements appear in nature is very important in Enem and in any entrance exam. The main topics relating to knowledge of substances and mixtures are:
Simple and compound substances
Types of mixtures
eutectic mixture
azeotropic mixture
4- Mixture separation methods
Mixture separation methods are used to carry out the separation of components (substances) from homogeneous and heterogeneous mixtures. The main methods of separation of mixtures are:
fractional dissolution
Decantation
filtration
simple distillation and fractional
fractional fusion
5- The study of the atom
The study of the atom involves all discoveries linked to the constitution of matter. The main topics related to the atomic study are:
Dalton's atomic model
Thomson Atomic Model
Rutherford Atomic Model
Bohr's atomic model
atom structure
Evolution of the atomic model
Eletronic distribution
ions
Atomic Similarities
6- Periodic table
Knowledge about chemical elements and their organization is fundamental to understanding how substances are formed. The main topics referring to the periodic table are:
Organization of the periodic table
Classification of chemical elements (metals, ametals, noble gases and hydrogen)
Periodic properties (atomic ray, ionization energy, electronegativity and electropositivity)
7- Chemical bonds
The study of chemical bonds allows us to get a sense of the behavior (solubility, melting point, boiling point, dissociation and ionization) of a given substance. The main topics regarding chemical bonds are:
ionic bonds
Characteristics of ionic compounds
covalent bonds
Characteristics of covalent compounds
Metal links
Metal alloys
8- Molecular geometry, polarity and intermolecular forces
Knowing the geometry, polarity and strength helps to understand important properties such as solubility, melting point and boiling point. The main topics are:
Types of Molecular Geometry (angular, linear, tetrahedral, trigonal and pyramidal)
Polar and non-polar molecules
Polar and non-polar connections
Intermolecular Force Induced Dipole
permanent dipole intermolecular strength
hydrogen bond
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/o-que-estudar-quimica-geral-para-enem.htm