The first radioactive chemical element to be discovered was uranium, by scientists Antoine Henri Becquerel (1852-1908), Marie Sklodowska Curie (1867-1934) and Pierre Curie (1859-1906). The discovery of radioactivity led them to win the Nobel Prize in Physics in 1903.
The Curies then proceeded to study radioactivity more deeply and to carry out a series of experiments with two uranium minerals – the pitchblende (uranium oxide) and chalcolite (copper and uranyl phosphate). However, what most caught their attention is that these ores were even more radioactive than the isolated metallic uranium, leading them to the conclusion that there would be another radioactive element present in the minerals.
They then began arduous work in order to separate the constituents of pitchblende, looking for the other element that could be contributing to the observed radiation. Scientists obtained, from the Austrian government, a ton of pitchblende, coming from the Joachimstal mines, located in Bohemia (Czech Republic). After three months, they managed to isolate a new radioactive element, polonium (named after Marie's homeland). However, the pure ore was still more radioactive than could be explained by the presence of polonium alone; therefore, the work continued.
In one of the two radioactive fractions they eventually managed to obtain was a new element, which they called “radio” (from the Latin radius, lightning), because it seems more radioactive than any other element. Today we know that radium is two million times more radioactive than uranium.
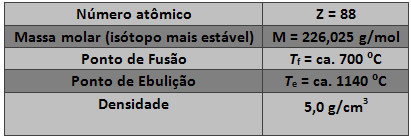
Some properties of this element are listed in the table below:
A spectroscopic analysis was carried out on the mixture of radium chloride that had been obtained and it was verified the appearance of a new line in the ultraviolet region (381.47 nm); which represented important proof of the discovery of radium.
But they still hadn't managed to isolate the radio; so the Curies started this task from a ton of pitchblende waste. After three years of consecutive work, with extreme patience and perseverance, the couple isolated 1 decigram of pure radium in 1902. It glowed in the dark and was always at a higher temperature than its surroundings.
The following year, Marie Curie received her second Nobel Prize (in Chemistry) for discovering radium and polonium, for isolating metallic radium, and for studying its compounds. She was the first person to receive two Nobel Prizes.
In 1908, Frederick Soddy (1877-1956) claimed that the energy released in the disintegration of radium was nearly a million times greater than that obtained by the same mass of matter subjected to any of the transformations known prior to the discovery of radioactivity. This made people start using this great source of energy for multiple purposes, such as: to cure dermatological problems, to fortify the organism, to clean objects and even to cure cancer.
Radium was even considered a miraculous substance with powers such as the ability to be responsible for generating life, to rejuvenate and revitalize the skin. It started to be used in facial treatments, for the elimination of wrinkles, acnes, blackheads, skin whitening and was incorporated into several products, such as skin creams. beauty, shampoos, soaps, bath salts, invigorating tonics (which were intended to recover and maintain mental, physical and sexual vigor), in articles physician-pharmacists prescribed against no less than 150 endocrinological disorders, pills, razors, toothpastes, compresses, "sources" of radioactive water, etc.

The misapplication of radio has led to many ills and even the death of many people. To cite an example, it was used in inks used in watch hands and dials. Women who applied this paint thinned their brushes in their mouths; with that, they swallowed small portions of radium. Within about two years, nine women died of severe anemia, accompanied by lesions in the mouth and jaw.
Marie Curie died in 1934, a victim of radiation to which she was exposed at work. But before that, she came to Brazil, in August 1926, where she visited Belo Horizonte and went to the “Instituto do Radium”, the first center dedicated to the fight against cancer in our country.
This unrestrained application of radium did not reach Brazil, due to the high cost of treatments with radium salts.
The “radio age” disappeared in the United States in the early 1930s; and in Europe it lasted until the beginning of World War II.
Today, radium is used in the treatment of some types of cancer, in instruments for detecting flaws in metallic objects and for geophysical prospecting for oil.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/radio-um-elemento-radioativo.htm
