The alkadienes or dienes are Hydrocarbons Open-chain (formed only by hydrogen and carbon atoms) that have two double bonds between carbons.
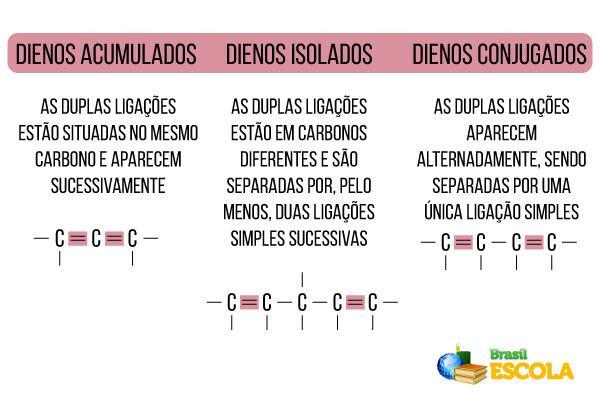
According to the location of the unsaturations, alkadienes can be classified as follows:
The nomenclature of alkadienes follows the same rule as alkenes nomenclature, with the only difference that instead of the infix being “en”, we will have the infix “dien”. "Di" refers to "two" and "en" refers to the presence of a double bond, that is, "two double bonds". So we have:

Examples:
CH3 ─ C ═ CH ─CHCH2: pent-1,3-diene - it is necessary to number where the two double bonds are coming from. Also note that the smallest possible numbers are used, and the count should be from the closest end of the double bond. It would be wrong if it were pent-2,4-diene.
H2C═C═CH2: propadiene - in this case it is not necessary to number because there is no other possibility of locating the unsaturations. Also, note that the vowel “a” was added between the prefix “prop” and the infix “dien” to make the word more phonetically correct.
H2C ═ CH ─ CH2 CH2 ─ CH ═ CH2: hex-1,5-diene
The general formula of alkadienes is ÇnoH2n-2, and “n” is any integer equal to or greater than 3. This is also the general formula for alkynes, which means it can occur function isomerism. For example, if n = 4, the corresponding alkadiene will have four carbons and six hydrogens (2. 4 – 2 = 6) and its formula will be:
H2C CH ─ CH ═ CH2
The alkyne with the molecular formula Ç4H6 will be given by:
HC ≡ C ─ CH2 CH3
In everyday life, the most important alkadians are called terpenes. Details about this group and its applications can be found in the text terpenes. But, in short, this is a group of compounds formed by the union of several molecules of isoprene, whose structure is shown below:
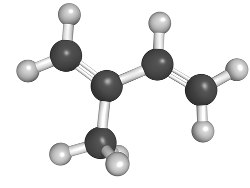
isoprene molecule
In nature, terpenes mainly comprise essential oils extracted from vegetables and fruits for the production of perfumes and flavoring agents. Among them, we have the limonene, an essential oil extracted from lemon and orange peels. It has the structure formed by the union of two isoprene molecules.
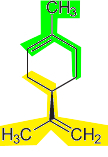
Limonene structure formed by two isoprene units shown in green and yellow colors
O beta carotene (vitamin A) present in carrots is responsible for its characteristic orange color and is formed by a system of conjugated double bonds:

Beta-carotene molecule present in carrots
Another very important example is the main constituent of latex extracted from rubber trees for the production of natural rubber. It is the polymer polyisoprene, formed by the repetition of isoprene units.
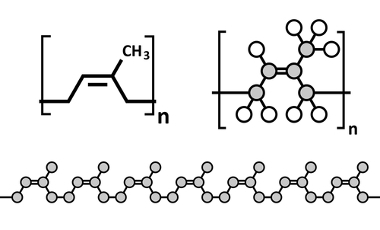
Representations of polyisoprene - constituent of natural rubber used in the manufacture of surgical gloves, condoms, boots, etc.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/alcadienos-ou-dienos.htm
